Titration Curve With Pka . Pka from the titration curve. I will show you how. For both titrations the titrant is 0.10 m naoh. Pka value can be determined by the titration curve. To calculate the pka of the solution, firstly, we will determine. As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. By the end of this section, you will be able to: And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. Sketch titration curves for the following two systems: K a corresponds to the. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph.
from www.numerade.com
To calculate the pka of the solution, firstly, we will determine. As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. Pka from the titration curve. K a corresponds to the. Pka value can be determined by the titration curve. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; Sketch titration curves for the following two systems: I will show you how. For both titrations the titrant is 0.10 m naoh.
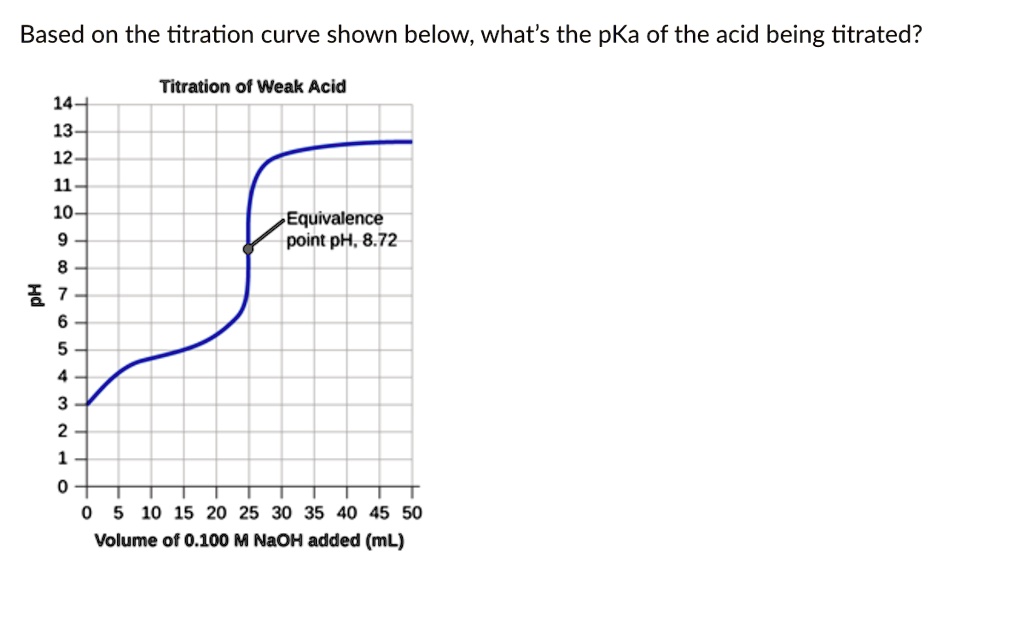
Based on the titration curve shown below, what's the pKa of the acid
Titration Curve With Pka K a corresponds to the. I will show you how. By the end of this section, you will be able to: And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. To calculate the pka of the solution, firstly, we will determine. Pka from the titration curve. For both titrations the titrant is 0.10 m naoh. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; Pka value can be determined by the titration curve. K a corresponds to the. As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. Sketch titration curves for the following two systems: In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph.
From thepeacechallenge.blogspot.com
Arginine Titration Curve Brain Mind Article Titration Curve With Pka By the end of this section, you will be able to: And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. I will show you how. To calculate the pka of the solution, firstly, we. Titration Curve With Pka.
From www.chegg.com
Solved Using the following data to graph titration curves. Titration Curve With Pka And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. Sketch titration curves for the following two systems: I will show you how. For both titrations the titrant is 0.10 m naoh. To calculate the. Titration Curve With Pka.
From mavink.com
Pka Titration Curve Titration Curve With Pka And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk. Titration Curve With Pka.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers Titration Curve With Pka By the end of this section, you will be able to: Pka from the titration curve. To calculate the pka of the solution, firstly, we will determine. In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. (a) the titration of 50.0 ml of 0.050 m h 2 a,. Titration Curve With Pka.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve With Pka Pka value can be determined by the titration curve. As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. By the end of this section, you will be able to: I will show you how. For both titrations the titrant is 0.10 m naoh. To calculate the. Titration Curve With Pka.
From ar.inspiredpencil.com
Curve Pka Titration Curve With Pka In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. Pka from the titration curve. As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. Sketch titration curves for the following two systems: For both titrations. Titration Curve With Pka.
From www.chegg.com
Solved Below is the titration curve for histidine. The pKa Titration Curve With Pka In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. By the end of this section, you will be able to: I will show you how. For both titrations the titrant is 0.10 m naoh. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic. Titration Curve With Pka.
From www.numerade.com
SOLVED Clearly draw the titration curve for the reaction of this amino Titration Curve With Pka By the end of this section, you will be able to: In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. For both titrations the titrant is 0.10 m naoh. I will show you how. As an acid, a polyprotic acids have a very small acid dissociation constant (ka. Titration Curve With Pka.
From www.reddit.com
[college chem] confused on how to get the concentration with the pH and Titration Curve With Pka Pka from the titration curve. K a corresponds to the. For both titrations the titrant is 0.10 m naoh. As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a. Titration Curve With Pka.
From solvedlib.com
Given the following titration curve estimate the pKa … SolvedLib Titration Curve With Pka Sketch titration curves for the following two systems: In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a. Titration Curve With Pka.
From www.numerade.com
SOLVED The pKa values for lysine are 2.2, 9.0 and 10.0. Draw a Titration Curve With Pka K a corresponds to the. To calculate the pka of the solution, firstly, we will determine. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; I will show you how. As an acid, a polyprotic acids have a very small acid. Titration Curve With Pka.
From www.chegg.com
Solved What is the approximate pKa of the weak acid titrated Titration Curve With Pka Pka value can be determined by the titration curve. K a corresponds to the. Pka from the titration curve. In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1. Titration Curve With Pka.
From www.youtube.com
pKa from a Titration Curve YouTube Titration Curve With Pka K a corresponds to the. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a. Titration Curve With Pka.
From 45.153.231.124
Titration Curves Reveal The Pka Of Weak Acids Amino Acids Gambaran Titration Curve With Pka Pka value can be determined by the titration curve. For both titrations the titrant is 0.10 m naoh. To calculate the pka of the solution, firstly, we will determine. In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. (a) the titration of 50.0 ml of 0.050 m h. Titration Curve With Pka.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve With Pka I will show you how. For both titrations the titrant is 0.10 m naoh. In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m. Titration Curve With Pka.
From www.reddit.com
in pH titration, how do you determine the (multiple) pKa points from Titration Curve With Pka And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. Pka value can be determined by the titration curve. K a corresponds to the. (a) the titration of 50.0 ml of 0.050 m h 2. Titration Curve With Pka.
From www.wizeprep.com
Titration Curves Wize University Chemistry Textbook Wizeprep Titration Curve With Pka And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. By the end of this section, you will be able to: In this video, i will teach you how to calculate the pka and the. Titration Curve With Pka.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve With Pka K a corresponds to the. Pka value can be determined by the titration curve. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. By the end of this section, you will be able to:. Titration Curve With Pka.
From mungfali.com
Acid Titration Curve Titration Curve With Pka For both titrations the titrant is 0.10 m naoh. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid. Titration Curve With Pka.
From www.vrogue.co
What Is Titration Curve What Is Pka Easybiologyclass vrogue.co Titration Curve With Pka Pka from the titration curve. K a corresponds to the. I will show you how. In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. Pka value can be determined by the titration curve. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak. Titration Curve With Pka.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid Titration Curve With Pka To calculate the pka of the solution, firstly, we will determine. I will show you how. By the end of this section, you will be able to: K a corresponds to the. Sketch titration curves for the following two systems: And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a. Titration Curve With Pka.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve With Pka (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. I will show you how. And (b) the titration of a. Titration Curve With Pka.
From www.vrogue.co
How To Draw A Titration Curve Given Pka Draw So Cute vrogue.co Titration Curve With Pka Sketch titration curves for the following two systems: I will show you how. Pka from the titration curve. To calculate the pka of the solution, firstly, we will determine. In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. As an acid, a polyprotic acids have a very small. Titration Curve With Pka.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve With Pka As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. To calculate the pka of the solution, firstly, we will determine. I will show you how. By the end of this section, you will be able to: K a corresponds to the. (a) the titration of 50.0. Titration Curve With Pka.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Curve With Pka Sketch titration curves for the following two systems: K a corresponds to the. Pka from the titration curve. For both titrations the titrant is 0.10 m naoh. Pka value can be determined by the titration curve. To calculate the pka of the solution, firstly, we will determine. By the end of this section, you will be able to: As an. Titration Curve With Pka.
From www.numerade.com
SOLVED Draw a titration curve for lactic acid (pKa = 3.86). Label the Titration Curve With Pka Sketch titration curves for the following two systems: K a corresponds to the. I will show you how. In this video, i will teach you how to calculate the pka and the ka simply from analysing a titration graph. To calculate the pka of the solution, firstly, we will determine. And (b) the titration of a 50.0 ml mixture containing. Titration Curve With Pka.
From chemistry.stackexchange.com
Titration of CH3COONa with HCl and pKa determination from half Titration Curve With Pka (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; I will show you how. Pka value can be determined by the titration curve. As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures. Titration Curve With Pka.
From www.chegg.com
Solved The graph shows the titration curves of a strong acid Titration Curve With Pka K a corresponds to the. Sketch titration curves for the following two systems: To calculate the pka of the solution, firstly, we will determine. I will show you how. By the end of this section, you will be able to: For both titrations the titrant is 0.10 m naoh. Pka value can be determined by the titration curve. (a) the. Titration Curve With Pka.
From www.researchgate.net
Titration curves of weak diacids with pK a1 = 4 and pK a2 = 5 or 5.3, C Titration Curve With Pka As an acid, a polyprotic acids have a very small acid dissociation constant (ka k a), which measures the strength of the acid. Pka from the titration curve. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; To calculate the pka. Titration Curve With Pka.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Titration Curve With Pka By the end of this section, you will be able to: Sketch titration curves for the following two systems: (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; As an acid, a polyprotic acids have a very small acid dissociation constant. Titration Curve With Pka.
From www.chegg.com
Solved Below you can find the titration curve for arginine. Titration Curve With Pka To calculate the pka of the solution, firstly, we will determine. Sketch titration curves for the following two systems: And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. As an acid, a polyprotic acids. Titration Curve With Pka.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Curve With Pka Sketch titration curves for the following two systems: K a corresponds to the. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. Pka from the titration curve. In this video, i will teach you. Titration Curve With Pka.
From www.youtube.com
Titration curves, pKa, pI, and Buffering regions YouTube Titration Curve With Pka And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. By the end of this section, you will be able to: For both titrations the titrant is 0.10 m naoh. Pka value can be determined. Titration Curve With Pka.
From japaneseclass.jp
PKA PKA JapaneseClass.jp Titration Curve With Pka K a corresponds to the. Pka value can be determined by the titration curve. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7; In this video, i will teach you how to calculate the pka and the ka simply from analysing. Titration Curve With Pka.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve With Pka Pka value can be determined by the titration curve. I will show you how. And (b) the titration of a 50.0 ml mixture containing 0.075 m ha, a weak acid with a pk a of 3, and 0.025 m hb, a weak acid with a pk a of 7. As an acid, a polyprotic acids have a very small acid. Titration Curve With Pka.