Titration Equivalence Point For Weak Acid . A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. equivalence point (v = 25 ml): titration curves for weak acid v weak base. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). It so happens that these two are both about. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The common example of this would be ethanoic acid and ammonia.
from general.chemistrysteps.com
The common example of this would be ethanoic acid and ammonia. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. It so happens that these two are both about. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. titration curves for weak acid v weak base. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. equivalence point (v = 25 ml):
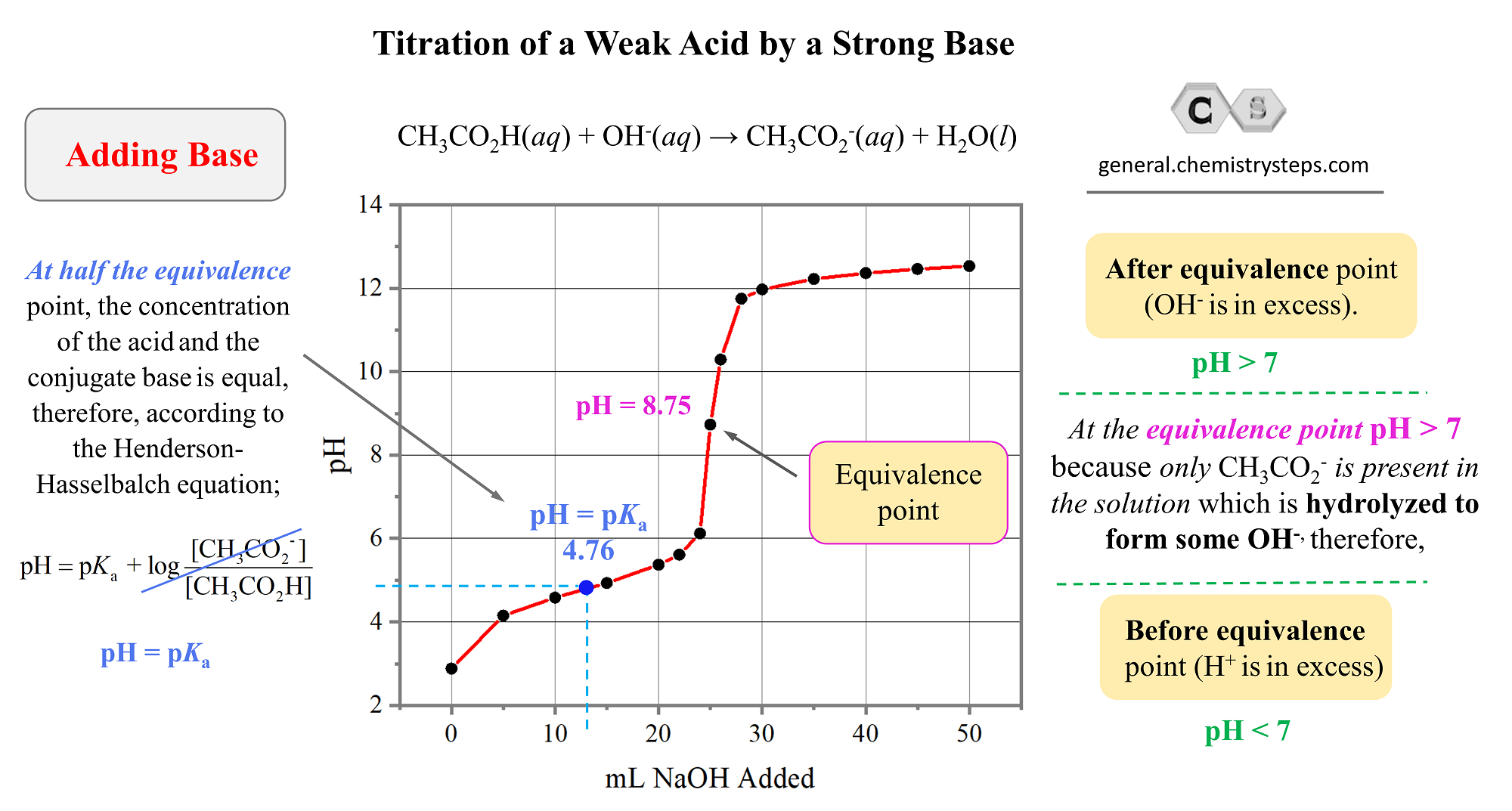
Titration of a Weak Acid by a Strong Base Chemistry Steps
Titration Equivalence Point For Weak Acid titration curves for weak acid v weak base. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. The common example of this would be ethanoic acid and ammonia. It so happens that these two are both about. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. titration curves for weak acid v weak base. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. equivalence point (v = 25 ml): the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Equivalence Point For Weak Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). titration curves for weak acid v weak base. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. The common example. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at Titration Equivalence Point For Weak Acid the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. The common example of this would be ethanoic acid and ammonia. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the titration of a weak acid with a. Titration Equivalence Point For Weak Acid.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Equivalence Point For Weak Acid The common example of this would be ethanoic acid and ammonia. titration curves for weak acid v weak base. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the equivalence point is defined as the point where th e moles of strong acid added = initial. Titration Equivalence Point For Weak Acid.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Titration Equivalence Point For Weak Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the equivalence point is defined as the point where th e. Titration Equivalence Point For Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Equivalence Point For Weak Acid titration curves for weak acid v weak base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an.. Titration Equivalence Point For Weak Acid.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Equivalence Point For Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The common example of this would be ethanoic acid and ammonia. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. It. Titration Equivalence Point For Weak Acid.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid Titration Equivalence Point For Weak Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). equivalence point (v = 25 ml): A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the equivalence point is. Titration Equivalence Point For Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Titration Equivalence Point For Weak Acid The common example of this would be ethanoic acid and ammonia. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. titration curves for weak acid v weak base. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube Titration Equivalence Point For Weak Acid equivalence point (v = 25 ml): the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. It so happens that these two are both about. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m. Titration Equivalence Point For Weak Acid.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Equivalence Point For Weak Acid equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong. Titration Equivalence Point For Weak Acid.
From www.writework.com
Titration of amino acids WriteWork Titration Equivalence Point For Weak Acid titration curves for weak acid v weak base. equivalence point (v = 25 ml): The common example of this would be ethanoic acid and ammonia. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. It so happens that these two are both. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Finding the pH during titration of a weak acid with a strong base, at Titration Equivalence Point For Weak Acid the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the titration of a weak acid with a strong base involves the. Titration Equivalence Point For Weak Acid.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Equivalence Point For Weak Acid It so happens that these two are both about. equivalence point (v = 25 ml): the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Equivalence Point For Weak Acid A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100. Titration Equivalence Point For Weak Acid.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers Titration Equivalence Point For Weak Acid It so happens that these two are both about. the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. The common example of this would be ethanoic acid and ammonia. titration curves for weak acid v weak base. the titration of a weak acid with a strong base involves. Titration Equivalence Point For Weak Acid.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Titration Equivalence Point For Weak Acid the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. The common example of this would be ethanoic acid and ammonia. It so happens that these two are both about. A drastic rise in ph is observed as the solution composition transitions from acidic. Titration Equivalence Point For Weak Acid.
From www.slideshare.net
pH Understanding titration curve Titration Equivalence Point For Weak Acid the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. It so happens that these two are both about. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration Equivalence Point For Weak Acid the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. A drastic rise in ph is observed as the solution composition transitions from. Titration Equivalence Point For Weak Acid.
From www.numerade.com
SOLVED Calculate the Ka of your weak acid using three different points Titration Equivalence Point For Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. titration curves for weak acid v weak base. the equivalence point is defined as. Titration Equivalence Point For Weak Acid.
From www.numerade.com
SOLVED 2) The titration curve for the titration of a weak acid with a Titration Equivalence Point For Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. The common example of this would be ethanoic acid and ammonia. A. Titration Equivalence Point For Weak Acid.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid Titration Equivalence Point For Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. equivalence point (v = 25 ml): the equivalence point is defined as. Titration Equivalence Point For Weak Acid.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Equivalence Point For Weak Acid equivalence point (v = 25 ml): the shapes of titration curves for weak acids and bases depend dramatically on the identity of the compound. It so happens that these two are both about. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. for the titration. Titration Equivalence Point For Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Equivalence Point For Weak Acid the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. titration curves for weak acid v weak base. The common example of this would be. Titration Equivalence Point For Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Titration Equivalence Point For Weak Acid titration curves for weak acid v weak base. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. The common example of this would be ethanoic acid and ammonia. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Titration Equivalence Point For Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. It so happens that these two are both about. The common example. Titration Equivalence Point For Weak Acid.
From fyoizufqj.blob.core.windows.net
Equivalence Point Amino Acid Titration at Christine Grosso blog Titration Equivalence Point For Weak Acid equivalence point (v = 25 ml): A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. The common example of this would be ethanoic acid and ammonia. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an. Titration Equivalence Point For Weak Acid.
From www.numerade.com
SOLVEDThe titration curves for two acids with the same base are(a Titration Equivalence Point For Weak Acid equivalence point (v = 25 ml): the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. The common example of this would be. Titration Equivalence Point For Weak Acid.
From www.numerade.com
SOLVED 1) Consider the following titration curve for a weak acid, X Jl Titration Equivalence Point For Weak Acid the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. It so happens that these two are both about. The common example of this would be ethanoic acid and ammonia. for the titration of a weak base with a strong acid, the ph. Titration Equivalence Point For Weak Acid.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Equivalence Point For Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the titration curve for the titration of 25.00 ml of 0.100 m ch. Titration Equivalence Point For Weak Acid.
From www.youtube.com
MCAT Acid/Base Equilibria Titration Curve of Weak Acid Strong Base Titration Equivalence Point For Weak Acid The common example of this would be ethanoic acid and ammonia. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b. titration curves for weak acid v weak base. equivalence point (v = 25 ml): the titration curve for the titration of 25.00 ml. Titration Equivalence Point For Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Equivalence Point For Weak Acid The common example of this would be ethanoic acid and ammonia. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. titration curves for weak acid v. Titration Equivalence Point For Weak Acid.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Equivalence Point For Weak Acid It so happens that these two are both about. equivalence point (v = 25 ml): equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the equivalence point is defined as the point where th e moles of strong acid added = initial moles of base b.. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Titration Weak base/strong acid Before the equivalence point YouTube Titration Equivalence Point For Weak Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The common example of this would be ethanoic. Titration Equivalence Point For Weak Acid.
From gregorycbroman.blob.core.windows.net
Titration Definition Wiki at gregorycbroman blog Titration Equivalence Point For Weak Acid titration curves for weak acid v weak base. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. The common example of this would be ethanoic acid. Titration Equivalence Point For Weak Acid.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration Equivalence Point For Weak Acid equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. equivalence point (v = 25 ml): The common example of this would be ethanoic acid and ammonia. It so happens that these two are both about. for the titration of a weak base with a strong acid,. Titration Equivalence Point For Weak Acid.