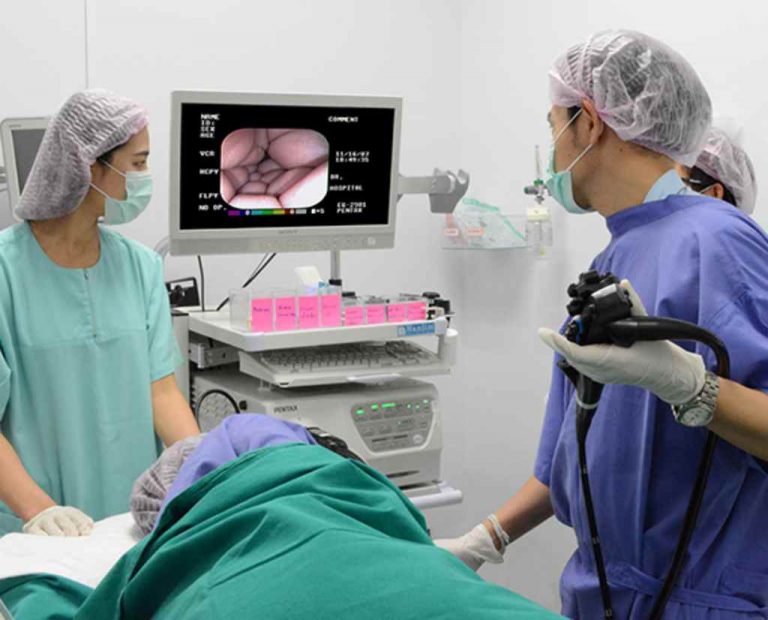
Endoscopy Unit Standards . Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. In 2022 endoscopy units must be. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. These range from recommendations on testing and screenings to the role of endoscopy. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Here you will find asge guidelines for standards of practice. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Food and drug administration [fda], 2015; Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the.
from doktersehat.com
In 2022 endoscopy units must be. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. These range from recommendations on testing and screenings to the role of endoscopy. Food and drug administration [fda], 2015; Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Here you will find asge guidelines for standards of practice. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and.
Endoskopi Prosedur, Efek Samping, & Biaya
Endoscopy Unit Standards In 2022 endoscopy units must be. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. These range from recommendations on testing and screenings to the role of endoscopy. Here you will find asge guidelines for standards of practice. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. In 2022 endoscopy units must be. Food and drug administration [fda], 2015; Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care.
From www.slideshare.net
Endoscopy Unit Endoscopy Unit Standards Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Food and drug administration [fda], 2015; Here you will find asge guidelines for standards of practice. These range from recommendations on testing and screenings to the role of endoscopy.. Endoscopy Unit Standards.
From journals.sagepub.com
Development of a national automated endoscopy database The United Kingdom National Endoscopy Endoscopy Unit Standards Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Food and drug administration [fda], 2015; Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1. Endoscopy Unit Standards.
From www.researchgate.net
Floor plans of endoscopy rooms from the case studies Download Scientific Diagram Endoscopy Unit Standards These range from recommendations on testing and screenings to the role of endoscopy. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Food and drug administration [fda], 2015; In 2022 endoscopy units must. Endoscopy Unit Standards.
From divinegracemedicalcenter.com
Digestive Endoscopy Unit Divine Grace Medical Center (DGMC) Hospital General Trias, Cavite Endoscopy Unit Standards Here you will find asge guidelines for standards of practice. In 2022 endoscopy units must be. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. These range from recommendations on testing and screenings to the role of endoscopy. Guidelines for appropriate use of endoscopy are based on a critical review. Endoscopy Unit Standards.
From www.esht.nhs.uk
Endoscopy East Sussex Healthcare NHS Trust Endoscopy Unit Standards Food and drug administration [fda], 2015; Here you will find asge guidelines for standards of practice. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. In 2022 endoscopy units must be. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in. Endoscopy Unit Standards.
From www.slideshare.net
Endoscopy Unit Endoscopy Unit Standards Food and drug administration [fda], 2015; Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes. Endoscopy Unit Standards.
From www.cghjournal.org
Improving and Driving Efficiency in Your Endoscopy Unit in the 21st Century Clinical Endoscopy Unit Standards Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Here you will find asge guidelines for standards of practice. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. These range from recommendations on testing and screenings to the role of endoscopy. The. Endoscopy Unit Standards.
From www.pinterest.com
Figure 1 Endoscopy safety checklist employed at St. Mark's Hospital. Safety checklist Endoscopy Unit Standards In 2022 endoscopy units must be. Food and drug administration [fda], 2015; Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Here you will find asge guidelines for standards of practice. Guidelines for appropriate. Endoscopy Unit Standards.
From www.giejournal.org
COVID19 in endoscopy Time to do more? Gastrointestinal Endoscopy Endoscopy Unit Standards Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Food and drug administration [fda], 2015; Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as. Endoscopy Unit Standards.
From palawancoophospital.com
Endoscopy Unit Palawan Coop Hospital Endoscopy Unit Standards In 2022 endoscopy units must be. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. These range from recommendations on testing and screenings to the role of endoscopy. Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. The most efficient endoscopy units, across all practice models, will. Endoscopy Unit Standards.
From www.dgmc.co.za
Endoscopy Unit Colorectal Unit Wits Donald Gordon Medical Centre Endoscopy Unit Standards Here you will find asge guidelines for standards of practice. These range from recommendations on testing and screenings to the role of endoscopy. In 2022 endoscopy units must be. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Guidelines for appropriate use of endoscopy are based on a. Endoscopy Unit Standards.
From mawarmedical.com
Endoscopy Unit Endoscopy Unit Standards These range from recommendations on testing and screenings to the role of endoscopy. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Reprocessing refers to the validated process of. Endoscopy Unit Standards.
From www.indiamart.com
Arthoscopy endoscopy unit Standard with freezing at Rs 125000 Arthroscopy Set in Haridwar ID Endoscopy Unit Standards Food and drug administration [fda], 2015; Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Here you will find asge guidelines for standards of practice. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Procedures undertaken. Endoscopy Unit Standards.
From aonemedical.ae
Endoscopy unit Aone Medical Equipment LLC Endoscopy Unit Standards Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Food and drug administration [fda], 2015; Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Here you will find asge guidelines for standards of practice. Procedures undertaken in an endoscopy unit may include. Endoscopy Unit Standards.
From neilcott.co.uk
St Endoscopy Unit Neilcott Construction Limited Endoscopy Unit Standards Food and drug administration [fda], 2015; Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Here you will find asge guidelines for standards of practice. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. In 2022 endoscopy units must be. These range. Endoscopy Unit Standards.
From www.fca-arch.com
387 USC Endoscopy — Fong & Chan Architects Endoscopy Unit Standards Food and drug administration [fda], 2015; The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. These range from recommendations on testing and screenings to the role of endoscopy. Guidelines for appropriate use of endoscopy are. Endoscopy Unit Standards.
From www.youtube.com
Our Most Advanced Endoscopy System EVIS X1 Gastroenterology OLYMPUS YouTube Endoscopy Unit Standards Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Here you will find asge guidelines for standards of practice. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. These range from recommendations on testing and screenings to the role of endoscopy.. Endoscopy Unit Standards.
From pbpegi.com
Clinical and Procedural Guidelines for the Gastrointestinal Endoscopy Unit during the Covid19 Endoscopy Unit Standards Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Food and drug administration [fda], 2015; Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health. Endoscopy Unit Standards.
From www.youtube.com
A day in the Endoscopy Unit A Patient's journey through Endoscopy Endoscopy Pathway NHS Endoscopy Unit Standards Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Here you will find asge guidelines for standards of practice. In 2022 endoscopy units must be. Hence, gi endoscopy units are now held to the same. Endoscopy Unit Standards.
From www.radiowestnorfolk.co.uk
New Endoscopy Unit Opens Radio West Norfolk Endoscopy Unit Standards Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Here you will find asge guidelines for standards of practice. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Food and drug administration [fda], 2015; Guidelines for appropriate use of endoscopy are based on a critical review of. Endoscopy Unit Standards.
From www.scopecare.com
Flexible Endoscope Repair Fiberscope Videoscope Colonoscope Cystoscope Endoscopy Unit Standards Here you will find asge guidelines for standards of practice. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Procedures undertaken in an endoscopy unit may include. Endoscopy Unit Standards.
From palawancoophospital.com
Endoscopy Unit Palawan Coop Hospital Endoscopy Unit Standards Here you will find asge guidelines for standards of practice. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. In 2022 endoscopy units must be. These range from recommendations on testing and screenings. Endoscopy Unit Standards.
From doktersehat.com
Endoskopi Prosedur, Efek Samping, & Biaya Endoscopy Unit Standards These range from recommendations on testing and screenings to the role of endoscopy. Here you will find asge guidelines for standards of practice. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Food and drug administration [fda], 2015; The most efficient endoscopy units, across all practice models, will. Endoscopy Unit Standards.
From www.researchgate.net
Endoscopy Unit Workflow Process Download Scientific Diagram Endoscopy Unit Standards Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. These range from recommendations on testing and screenings to the role of endoscopy. Here you will find asge guidelines for standards of practice. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Guidelines. Endoscopy Unit Standards.
From slideplayer.com
HUROTELEMED project Department of Gastroenterology and Hepatology ppt download Endoscopy Unit Standards Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Here you. Endoscopy Unit Standards.
From abdominalkey.com
How Endoscopes Work Abdominal Key Endoscopy Unit Standards Food and drug administration [fda], 2015; Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. In 2022 endoscopy units must be. Reprocessing refers to the validated process. Endoscopy Unit Standards.
From www.researchgate.net
(PDF) The structure and delivery of a novel training course on endoscope reprocessing and Endoscopy Unit Standards Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. In 2022 endoscopy units must be. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Hence, gi endoscopy units. Endoscopy Unit Standards.
From www.researchgate.net
General endoscopy unit characteristics and reprocessing performance a Download Scientific Diagram Endoscopy Unit Standards Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating. Endoscopy Unit Standards.
From www.nnuh.nhs.uk
Norfolk and Norwich University Hospitals NHS Foundation Trust » New endoscopy unit praised for Endoscopy Unit Standards Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Food and drug administration [fda], 2015; In 2022 endoscopy units must be. The most efficient endoscopy units, across all practice models, will successfully navigate. Endoscopy Unit Standards.
From www.youtube.com
A guide to the new Endoscopy Suite at Chesterfield Royal Hospital NHS Foundation Trust YouTube Endoscopy Unit Standards Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Hence, gi endoscopy units are now held to. Endoscopy Unit Standards.
From www.tungshin.com.my
Endoscopy Unit Tung Shin Hospital Endoscopy Unit Standards Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. In 2022 endoscopy units must be. Guidelines for appropriate use of endoscopy are based on a critical review of the available data and expert consensus at the. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1. Endoscopy Unit Standards.
From www.researchgate.net
(PDF) The University Endoscopy Unit The Standard of Care? Endoscopy Unit Standards These range from recommendations on testing and screenings to the role of endoscopy. Food and drug administration [fda], 2015; Here you will find asge guidelines for standards of practice. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Reprocessing refers to the validated process of cleaning and then disinfecting or. Endoscopy Unit Standards.
From www.researchgate.net
(PDF) Proposed Standards for Canadian Endoscopy Units in the ’90s Endoscopy Unit Standards Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. In 2022 endoscopy units must be. These range from recommendations on testing and screenings to the role of endoscopy. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Here you will find. Endoscopy Unit Standards.
From mediyug.co.in
Portable Mobile Endoscopy Unit 3 In 1(Standard) Endoscopy Unit Standards Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. Procedures undertaken in an endoscopy unit may include gastrointestinal endoscopy (such as gastroscopy, colonoscopy, ercp (endoscopic. These range from recommendations on testing and screenings to the role of endoscopy. Guidelines for appropriate use of endoscopy are based on a critical review of the available data. Endoscopy Unit Standards.
From www.researchgate.net
Comparative Analysis between Endoscopy Quality Evaluation Criteria in... Download Table Endoscopy Unit Standards Reprocessing refers to the validated process of cleaning and then disinfecting or sterilizing endoscopes and. The most efficient endoscopy units, across all practice models, will successfully navigate the dynamic changes occurring in health care. Hence, gi endoscopy units are now held to the same standards as sterile operating rooms by cms1 without evidence demonstrating that. Food and drug administration [fda],. Endoscopy Unit Standards.