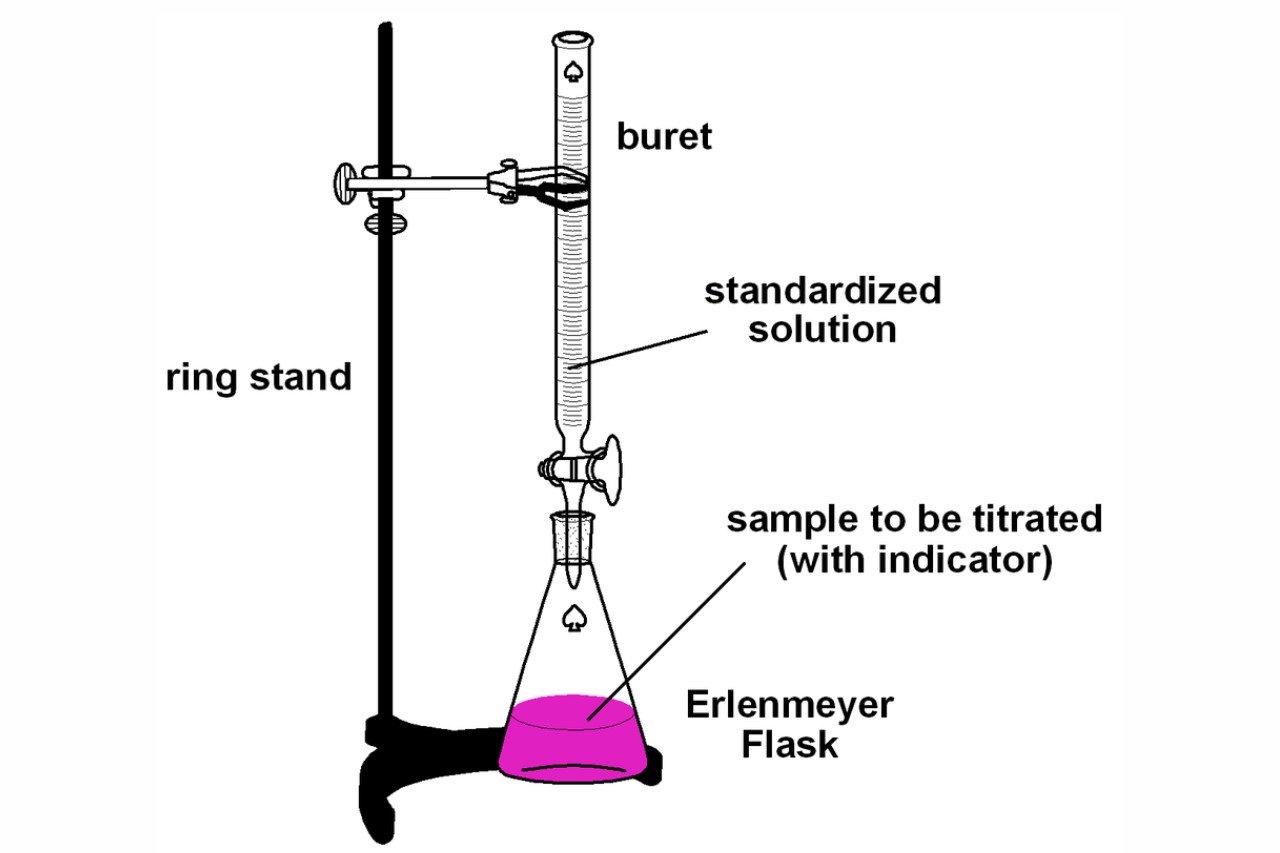
Titration Definition Simple . It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out. By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. For example, you can use it to find the concentration of a solution of an acid. Titration is a type of chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. A method or the activity of finding exactly how much of a substance there is in a solution by…. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. We consider it to be analysis because we use it to make a measurement.
from facts.net
It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. A method or the activity of finding exactly how much of a substance there is in a solution by…. By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out. We consider it to be analysis because we use it to make a measurement. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. For example, you can use it to find the concentration of a solution of an acid. Titration is a type of chemical analysis.
8 Captivating Facts About AcidBase Titration
Titration Definition Simple titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. We consider it to be analysis because we use it to make a measurement. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration is a type of chemical analysis. A method or the activity of finding exactly how much of a substance there is in a solution by…. For example, you can use it to find the concentration of a solution of an acid.
From exyxyiezl.blob.core.windows.net
Titration Tool Definition at Alicia Pelletier blog Titration Definition Simple titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. . Titration Definition Simple.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Definition Simple titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. We consider it to be analysis because we use it to make a measurement. A method or the activity of finding exactly how much of a substance there is in a solution by…. By this process, the acid. Titration Definition Simple.
From giofzfwwv.blob.core.windows.net
Acid Base Titration To Determine The Concentration at Latonya John blog Titration Definition Simple It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. For example, you can use it to find the concentration of a solution of an acid. Titration is a type of chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration. Titration Definition Simple.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Definition Simple in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out. For example, you can use it to find the concentration of a solution of an acid. titration is the process in which one solution is added to another solution such that it reacts under conditions in. Titration Definition Simple.
From giohqmnus.blob.core.windows.net
Titration Definition Wikipedia at James Henrich blog Titration Definition Simple For example, you can use it to find the concentration of a solution of an acid. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration involves the gradual addition of a reagent of known concentration, known as the. Titration Definition Simple.
From fyoxsyypa.blob.core.windows.net
In Titration What Goes In Burette Acid Or Base at Douglas Cox blog Titration Definition Simple titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution. Titration Definition Simple.
From ar.inspiredpencil.com
Titration Diagram Titration Definition Simple We consider it to be analysis because we use it to make a measurement. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. For example, you can use it to find the concentration of a solution of an acid. in a titration, a solution. Titration Definition Simple.
From giotsjinw.blob.core.windows.net
Titration Definition In Short at Angel Serrano blog Titration Definition Simple titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. titration is a. Titration Definition Simple.
From www.youtube.com
titration part1 definition YouTube Titration Definition Simple A method or the activity of finding exactly how much of a substance there is in a solution by…. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use it to find the concentration of a solution of an acid. titration is a quantitative. Titration Definition Simple.
From exyjmshzl.blob.core.windows.net
La Titration Definition at Abraham Godsey blog Titration Definition Simple Titration is a type of chemical analysis. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out. titration is the slow addition. Titration Definition Simple.
From www.thoughtco.com
Titration Definition (Chemistry) Titration Definition Simple titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. By this process, the acid. Titration Definition Simple.
From giotsjinw.blob.core.windows.net
Titration Definition In Short at Angel Serrano blog Titration Definition Simple Titration is a type of chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in quantitative analytical chemistry to determine an. Titration Definition Simple.
From fyoqeosdv.blob.core.windows.net
Titration Definition Medical Terms at Mercedes Ware blog Titration Definition Simple titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. For example, you can use it to find the concentration of a solution of an acid. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration involves the gradual. Titration Definition Simple.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Definition Simple titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration is a type of chemical analysis. A method or the activity of finding exactly how much of a substance there is in a solution by…. in a titration, a solution of unknown concentration is reacted with. Titration Definition Simple.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Titration Definition Simple in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration. Titration Definition Simple.
From exyxyiezl.blob.core.windows.net
Titration Tool Definition at Alicia Pelletier blog Titration Definition Simple titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. It is used in quantitative analytical chemistry to determine an unknown. Titration Definition Simple.
From giotwzxiy.blob.core.windows.net
Residual Titration Definition at Mary Jeffries blog Titration Definition Simple It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop. Titration Definition Simple.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Definition Simple By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. A method or the activity of finding exactly how much of a substance there is in a solution by…. We consider it to be analysis because we use it to make a measurement. For example, you can use it. Titration Definition Simple.
From exydnzhop.blob.core.windows.net
Laboratory Analysis Def at Juan Butler blog Titration Definition Simple By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration is a type of chemical analysis. For example, you can use it to find the concentration of a solution of an acid.. Titration Definition Simple.
From tuitiontube.com
Details about AcidBase Titration Lab in Easy Language Tuition Tube Titration Definition Simple A method or the activity of finding exactly how much of a substance there is in a solution by…. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration is a type of chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration Definition Simple.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Definition Simple Titration is a type of chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in quantitative analytical chemistry to determine an. Titration Definition Simple.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Definition Simple For example, you can use it to find the concentration of a solution of an acid. By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out.. Titration Definition Simple.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Definition Simple titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. A method or the activity of finding exactly how much of. Titration Definition Simple.
From gioumwmwd.blob.core.windows.net
Titration Meaning In Marathi at Linda blog Titration Definition Simple It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is a type of chemical analysis. titration is the slow addition of one solution of. Titration Definition Simple.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Definition Simple titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. By this process, the acid or base of a known concentration completely. Titration Definition Simple.
From ubicaciondepersonas.cdmx.gob.mx
Titration Equipment Kit ubicaciondepersonas.cdmx.gob.mx Titration Definition Simple titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. A method or the activity of finding exactly how much. Titration Definition Simple.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Definition Simple titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is a quantitative analysis to determine the concentration of an. Titration Definition Simple.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Definition Simple titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order. Titration Definition Simple.
From giotwzxiy.blob.core.windows.net
Residual Titration Definition at Mary Jeffries blog Titration Definition Simple By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. For example, you can use it to find the concentration of a solution of an acid. Titration is a type of chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample. Titration Definition Simple.
From giotsjinw.blob.core.windows.net
Titration Definition In Short at Angel Serrano blog Titration Definition Simple For example, you can use it to find the concentration of a solution of an acid. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. We consider it to be analysis because we use it to make a measurement. By this process, the acid or base of a known concentration. Titration Definition Simple.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Definition Simple A method or the activity of finding exactly how much of a substance there is in a solution by…. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in. Titration Definition Simple.
From giotwzxiy.blob.core.windows.net
Residual Titration Definition at Mary Jeffries blog Titration Definition Simple We consider it to be analysis because we use it to make a measurement. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. For example, you can use it to find the concentration of a solution of an acid. By this process, the acid or base of. Titration Definition Simple.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Definition Simple titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. A method or the activity of finding. Titration Definition Simple.
From www.youtube.com
Titration How to perform Titration Experiment/Practical YouTube Titration Definition Simple in a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out. We consider it to be analysis because we use it to make a measurement. Titration is a type of chemical analysis. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte.. Titration Definition Simple.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Definition Simple titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of. Titration Definition Simple.