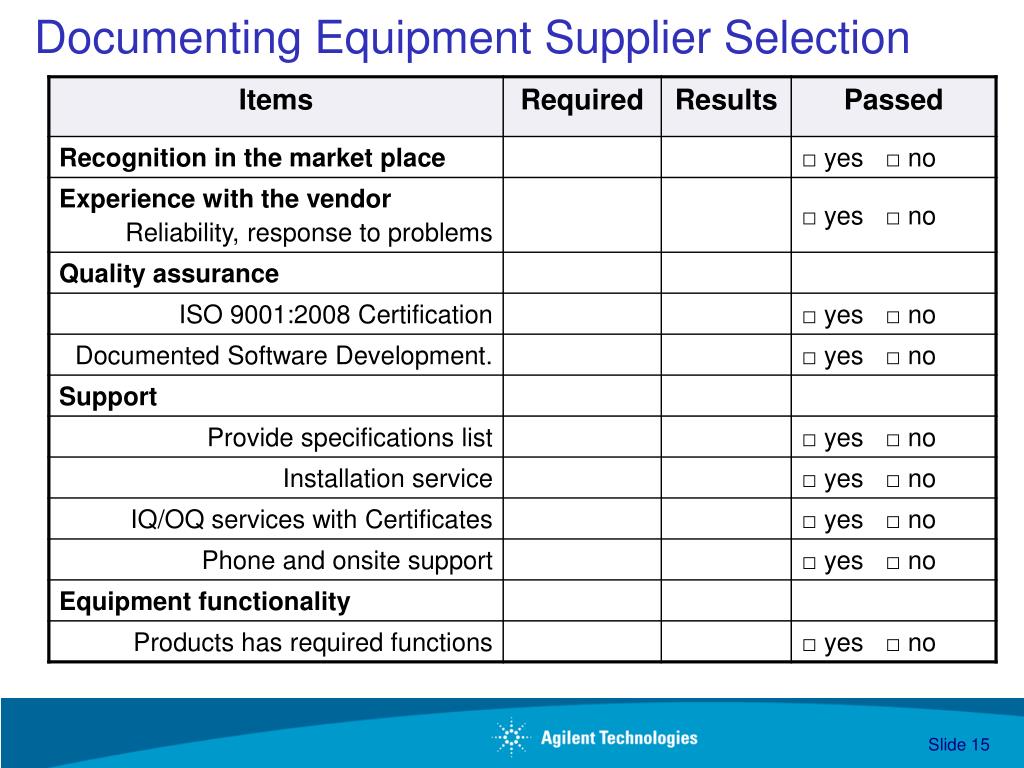
Gmp Equipment Validation Guidelines . Validation, as published in the world health organization (who). This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Organising and planning for qualification and validation. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Further to the supplementary guidelines on good manufacturing practices: Guidelines on good manufacturing practice specific to. 1.1 all qualification and validation activities should be.
from www.slideserve.com
Further to the supplementary guidelines on good manufacturing practices: This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Guidelines on good manufacturing practice specific to. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Organising and planning for qualification and validation. 1.1 all qualification and validation activities should be. Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation Validation, as published in the world health organization (who).
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and
Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. Further to the supplementary guidelines on good manufacturing practices: Validation, as published in the world health organization (who). Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation Guidelines on good manufacturing practice specific to. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Organising and planning for qualification and validation. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. 1.1 all qualification and validation activities should be.
From qvalon.com
The Complete Guide to Good Manufacturing Practices (GMP) by QVALON QVALON Gmp Equipment Validation Guidelines Organising and planning for qualification and validation. Guidelines on good manufacturing practice specific to. Further to the supplementary guidelines on good manufacturing practices: Validation, as published in the world health organization (who). This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. 1.1 all qualification and validation activities should be. The cleaning validation protocol. Gmp Equipment Validation Guidelines.
From www.inmedpharma.com
What are the GMP manufacturing standards for different products? Gmp Equipment Validation Guidelines Guidelines on good manufacturing practice specific to. Organising and planning for qualification and validation. Further to the supplementary guidelines on good manufacturing practices: Validation, as published in the world health organization (who). The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Facilities and equipment cgmp highlights • aseptic manufacturing. Gmp Equipment Validation Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Guidelines on good manufacturing practice specific to. Organising and planning for qualification and validation. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Further to. Gmp Equipment Validation Guidelines.
From www.kewaunee.in
Designing GMP Facilities to Meet Pharma Regulatory Standards Kewaunee Gmp Equipment Validation Guidelines Validation, as published in the world health organization (who). Organising and planning for qualification and validation. Guidelines on good manufacturing practice specific to. Further to the supplementary guidelines on good manufacturing practices: 1.1 all qualification and validation activities should be. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting. Gmp Equipment Validation Guidelines.
From loetgundi.blob.core.windows.net
Gmp Cleaning Validation Protocol at Beverly Norris blog Gmp Equipment Validation Guidelines The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation Organising and planning for qualification and validation. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and. Gmp Equipment Validation Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Guidelines on good manufacturing practice specific. Gmp Equipment Validation Guidelines.
From gmptemplates.com
E038001 EQUIPMENT QUALIFICATION PROCEDURE GMP Templates Gmp Equipment Validation Guidelines Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation 1.1 all qualification and validation activities should be. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Guidelines. Gmp Equipment Validation Guidelines.
From www.gmp-verlag.de
GMP LOGFILE Leitartikel GMPVerlag Acceptance testing or qualification? Gmp Equipment Validation Guidelines Organising and planning for qualification and validation. 1.1 all qualification and validation activities should be. Validation, as published in the world health organization (who). Guidelines on good manufacturing practice specific to. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Further to the supplementary guidelines on good manufacturing practices: The cleaning validation protocol. Gmp Equipment Validation Guidelines.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Validation, as published in the world health organization (who). This guidance aligns process validation activities with a product lifecycle concept and with. Gmp Equipment Validation Guidelines.
From www.uaeiso.org
GMP Certification Check Benefits, Safety & Integrity Ascent Gmp Equipment Validation Guidelines Validation, as published in the world health organization (who). The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Further to the supplementary guidelines on good manufacturing practices: This guidance aligns process validation activities with a product lifecycle concept and. Gmp Equipment Validation Guidelines.
From www.vietfil.com
What is GMP standard in pharmaceutical manufacturing Gmp Equipment Validation Guidelines The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Further to the supplementary guidelines on good manufacturing practices: This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Validation, as published in the world health organization (who). 1.1 all qualification and validation activities. Gmp Equipment Validation Guidelines.
From quallpharma.blogspot.com
Quallpharma consultancy The GMP Systems (General Requirement) Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. Further to the supplementary guidelines on good manufacturing practices: Guidelines on good manufacturing practice specific to. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting. Gmp Equipment Validation Guidelines.
From gmp.com.vn
WHO GMP guidelines Equipment Gmp Equipment Validation Guidelines The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Organising and planning for qualification and validation. Guidelines on good manufacturing practice specific to. Facilities and equipment cgmp highlights • aseptic manufacturing facility •. Gmp Equipment Validation Guidelines.
From studylib.net
A WHO guide to good manufacturing practice (GMP) requirements Part 2 Gmp Equipment Validation Guidelines The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Organising and planning for qualification and validation. Validation, as published in the world health organization (who). Further to the supplementary guidelines on good manufacturing practices: 1.1 all qualification and validation activities should be. The aim of this validation guideline is. Gmp Equipment Validation Guidelines.
From gmpinsiders.com
GMP Insiders Your Trusted Source For GMP Excellence! Gmp Equipment Validation Guidelines This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Guidelines on good manufacturing practice specific to. 1.1 all qualification and validation activities should be. Further to the supplementary guidelines on good manufacturing practices: Validation, as published in the world health organization (who). The cleaning validation protocol should describe the equipment to be cleaned,. Gmp Equipment Validation Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Gmp Equipment Validation Guidelines Further to the supplementary guidelines on good manufacturing practices: Validation, as published in the world health organization (who). Guidelines on good manufacturing practice specific to. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be. Gmp Equipment Validation Guidelines.
From slidetodoc.com
Basic Principles of GMP Qualification and Validation Section Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. Organising and planning for qualification and validation. Validation, as published in the world health organization (who). Further to the supplementary guidelines on good manufacturing practices: Guidelines on good manufacturing practice specific to. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The aim of this. Gmp Equipment Validation Guidelines.
From present5.com
Basic Principles of GMP Qualification and Validation Section Gmp Equipment Validation Guidelines The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. 1.1 all qualification and validation activities should be. Validation, as published in the world health organization (who). Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation The aim of this validation guideline is to. Gmp Equipment Validation Guidelines.
From www.scribd.com
Basic GMP Checklist For Pharmaceutical Plants PDF Verification And Gmp Equipment Validation Guidelines The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Further to the supplementary guidelines on good manufacturing practices: This. Gmp Equipment Validation Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Gmp Equipment Validation Guidelines The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation 1.1 all qualification and validation activities should be. Organising and planning for qualification and validation.. Gmp Equipment Validation Guidelines.
From pharmaanalytic.com
Equipment Qualification PharmaAnalytic LLC GMP, Quality Management Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Guidelines on good manufacturing practice specific to. Further to the supplementary guidelines on good manufacturing practices: Validation, as published in the world health organization (who). This guidance aligns process validation activities with. Gmp Equipment Validation Guidelines.
From angstromtechnology.com
What Do the GMP Qualification & Validation Processes Look Like Gmp Equipment Validation Guidelines This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Further to the supplementary guidelines on good manufacturing practices: Validation, as published in the world health organization (who). The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the. Gmp Equipment Validation Guidelines.
From gmp.com.vn
Comparison of the requirements of EU GMP guidelines versus WHO GMP Gmp Equipment Validation Guidelines This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Further to the supplementary guidelines on good manufacturing practices: The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. 1.1 all qualification and validation activities should be. Validation, as published in the world health. Gmp Equipment Validation Guidelines.
From present5.com
Basic Principles of GMP Qualification and Validation Section Gmp Equipment Validation Guidelines Further to the supplementary guidelines on good manufacturing practices: Guidelines on good manufacturing practice specific to. Validation, as published in the world health organization (who). The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Organising and planning for qualification and validation. The aim of this validation guideline is to. Gmp Equipment Validation Guidelines.
From www.studypool.com
SOLUTION LPA guidance GMP process validation Studypool Gmp Equipment Validation Guidelines This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Validation, as published in the world health organization (who). Organising and planning for qualification. Gmp Equipment Validation Guidelines.
From present5.com
Basic Principles of GMP Qualification and Validation Section Gmp Equipment Validation Guidelines 1.1 all qualification and validation activities should be. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Organising and planning for qualification and validation. Guidelines on good manufacturing practice specific to. The aim of this validation guideline is to provide a clear statement of the scope, validation approach and. Gmp Equipment Validation Guidelines.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and Gmp Equipment Validation Guidelines Organising and planning for qualification and validation. Guidelines on good manufacturing practice specific to. Further to the supplementary guidelines on good manufacturing practices: The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. The aim of this validation guideline is to provide a clear statement of the scope, validation approach. Gmp Equipment Validation Guidelines.
From isosrilanka.ascentworld.com
GMP Certification Good Manufacturing Practice Ascent Gmp Equipment Validation Guidelines The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Validation, as published in the world health organization (who). 1.1 all qualification and validation activities should be. Further to the supplementary guidelines on good manufacturing practices: Guidelines on good manufacturing practice specific to. The aim of this validation guideline is. Gmp Equipment Validation Guidelines.
From gmp.com.vn
WHO GMP guidelines Qualification and Validation Gmp Equipment Validation Guidelines Validation, as published in the world health organization (who). This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Guidelines on good manufacturing practice specific to. Further to the supplementary guidelines on good manufacturing practices: Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation The aim of this. Gmp Equipment Validation Guidelines.
From www.slideserve.com
PPT Qualification and Validation PowerPoint Presentation, free Gmp Equipment Validation Guidelines Guidelines on good manufacturing practice specific to. Further to the supplementary guidelines on good manufacturing practices: The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. Validation, as published in the world health organization (who). Organising and planning for qualification. Gmp Equipment Validation Guidelines.
From present5.com
Basic Principles of GMP Qualification and Validation Section Gmp Equipment Validation Guidelines This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. Further to the supplementary guidelines on good manufacturing practices: The aim of this validation guideline is to provide a clear statement of the scope,. Gmp Equipment Validation Guidelines.
From www.yumpu.com
Understanding Facility Validation for GMP compliance Indian Gmp Equipment Validation Guidelines The aim of this validation guideline is to provide a clear statement of the scope, validation approach and tes\ ting requirements for the validation of the equipment that is involved,. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Further to the supplementary guidelines on good manufacturing practices: Facilities and equipment cgmp highlights. Gmp Equipment Validation Guidelines.
From www.presentationeze.com
FDA GMP QSR Equipment and Maintenance PresentationEZE Gmp Equipment Validation Guidelines Facilities and equipment cgmp highlights • aseptic manufacturing facility • equipment qualification • cleaning validation Further to the supplementary guidelines on good manufacturing practices: Guidelines on good manufacturing practice specific to. Organising and planning for qualification and validation. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Validation, as published in the world. Gmp Equipment Validation Guidelines.
From www.studypool.com
SOLUTION LPA guidance GMP process validation Studypool Gmp Equipment Validation Guidelines Guidelines on good manufacturing practice specific to. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. The aim of this validation guideline is to provide a clear statement of the scope, validation approach. Gmp Equipment Validation Guidelines.
From present5.com
Basic Principles of GMP Qualification and Validation Section Gmp Equipment Validation Guidelines Guidelines on good manufacturing practice specific to. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance,. Validation, as published in the world health organization (who). Organising and planning for qualification and validation. The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, parameters to be monitored. 1.1. Gmp Equipment Validation Guidelines.