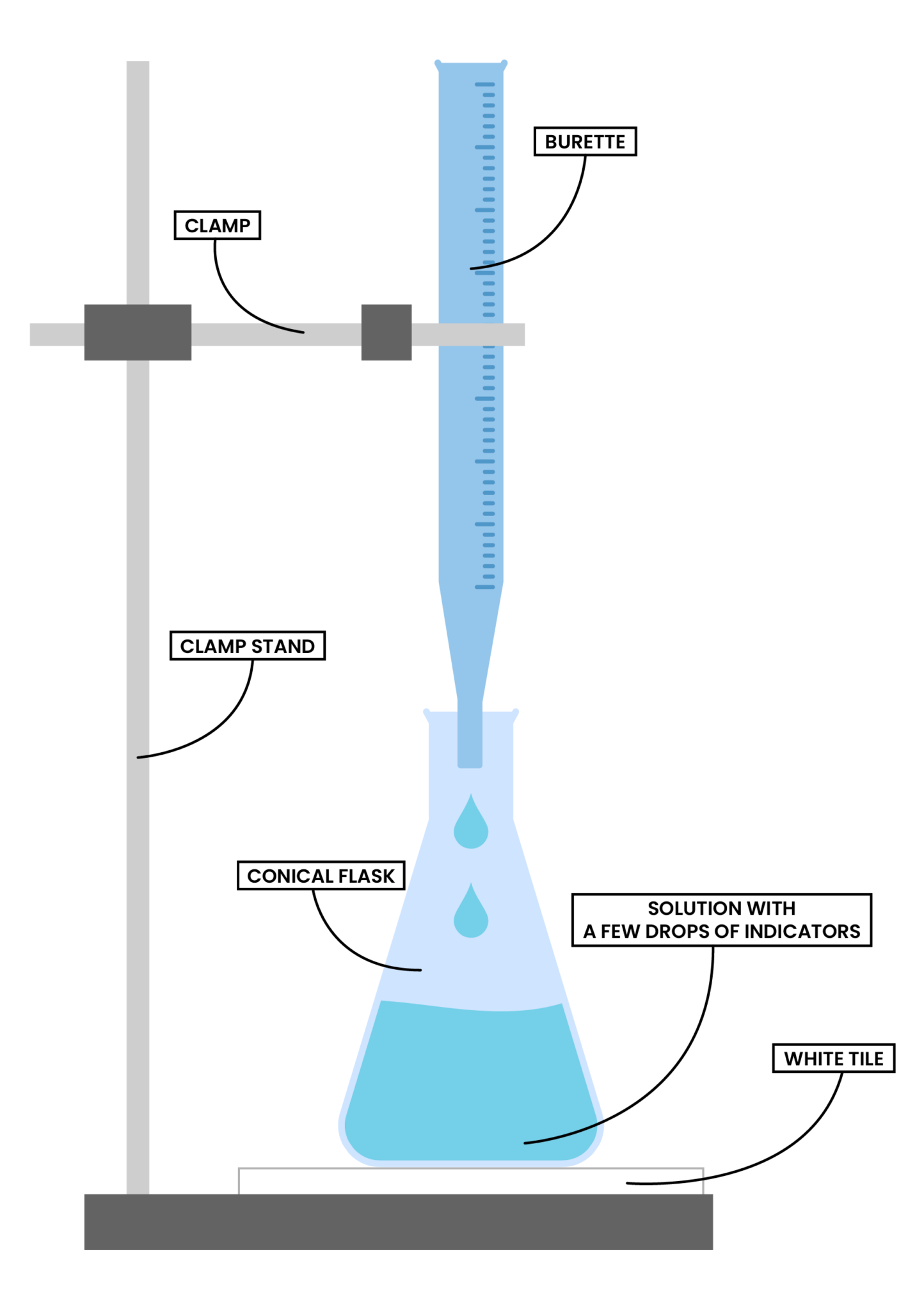
Titration In Chemistry Sentence . What is the equivalence point of a titration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Every titration uses the same players: how titrations work and what you need. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Learn the titration graph and titration equation. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. What are titrant and analyte.
from resource.studiaacademy.com
Every titration uses the same players: a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. What is the equivalence point of a titration. how titrations work and what you need. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What are titrant and analyte. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
Acids, Alkalis and Titrations Studia Academy Resources
Titration In Chemistry Sentence What are titrant and analyte. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. how titrations work and what you need. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Every titration uses the same players: What is the equivalence point of a titration. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Learn the titration graph and titration equation. What are titrant and analyte.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titration In Chemistry Sentence a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Every titration uses the same players: What is the equivalence point of a titration. a titration is a laboratory. Titration In Chemistry Sentence.
From mungfali.com
Acid Base Titration Procedure Titration In Chemistry Sentence What is the equivalence point of a titration. how titrations work and what you need. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration In Chemistry Sentence.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration In Chemistry Sentence how titrations work and what you need. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. Learn the titration graph and titration equation. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is a laboratory. Titration In Chemistry Sentence.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration In Chemistry Sentence titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is a laboratory technique used to precisely measure molar concentration. Titration In Chemistry Sentence.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration In Chemistry Sentence The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. performing chemical reactions quantitatively to determine the exact amount of a reagent. Titration In Chemistry Sentence.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration In Chemistry Sentence Every titration uses the same players: titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. how titrations work and what you need. The basic process involves. Titration In Chemistry Sentence.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration In Chemistry Sentence What is the equivalence point of a titration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What are titrant and analyte. titration, process. Titration In Chemistry Sentence.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration In Chemistry Sentence titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Every titration uses the same players: how titrations work and what you need. What is the equivalence point of a titration. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments. Titration In Chemistry Sentence.
From www.youtube.com
Analytical Chemistry 1 Volhard Method back titration (Slid 164166 Titration In Chemistry Sentence performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. titration, process of chemical analysis in which the quantity of some constituent of a. Titration In Chemistry Sentence.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration In Chemistry Sentence performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What is the equivalence point of a titration. The basic process involves adding a standard solution of one reagent to a known. Titration In Chemistry Sentence.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration In Chemistry Sentence titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Learn the titration graph and titration equation. What is the equivalence point of a titration. The basic process. Titration In Chemistry Sentence.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration In Chemistry Sentence how titrations work and what you need. Learn the titration graph and titration equation. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is defined as ‘the. Titration In Chemistry Sentence.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration In Chemistry Sentence The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Every titration uses the same players: titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is defined as ‘the. Titration In Chemistry Sentence.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration In Chemistry Sentence titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. What is the equivalence point of a titration. What are titrant and analyte. titration is the slow addition of one solution of a known. Titration In Chemistry Sentence.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration In Chemistry Sentence What is the equivalence point of a titration. Every titration uses the same players: What are titrant and analyte. Learn the titration graph and titration equation. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration, process of chemical analysis in which the quantity of some constituent. Titration In Chemistry Sentence.
From exymjkekq.blob.core.windows.net
Titration In Chemistry Equipment at Barbara Adamson blog Titration In Chemistry Sentence The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration In Chemistry Sentence.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Titration In Chemistry Sentence What are titrant and analyte. Learn the titration graph and titration equation. What is the equivalence point of a titration. how titrations work and what you need. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. titration, process of chemical analysis in which the. Titration In Chemistry Sentence.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. Titration In Chemistry Sentence titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. how titrations work and what you need. Learn the titration graph and titration equation. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is. Titration In Chemistry Sentence.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration In Chemistry Sentence how titrations work and what you need. What is the equivalence point of a titration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Every titration uses the same players: a titration is defined as ‘the process of determining the quantity of a substance a by. Titration In Chemistry Sentence.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. Titration In Chemistry Sentence The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. What is the equivalence point of a titration. Learn the titration graph and titration equation. titration, process of chemical. Titration In Chemistry Sentence.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration In Chemistry Sentence titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Every titration uses the same players: The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What is the equivalence point of a titration. a titration is a. Titration In Chemistry Sentence.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration In Chemistry Sentence What are titrant and analyte. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. What is the equivalence point of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. . Titration In Chemistry Sentence.
From letitsnowglobe.co.uk
Titration procedure pdf Titration In Chemistry Sentence The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments. Titration In Chemistry Sentence.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration In Chemistry Sentence how titrations work and what you need. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. performing chemical reactions quantitatively to determine the exact amount of a reagent. Titration In Chemistry Sentence.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration In Chemistry Sentence What are titrant and analyte. how titrations work and what you need. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Learn the titration graph and titration equation. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance.. Titration In Chemistry Sentence.
From parholikhotoacknoledge.blogspot.com
Titrations in Chemistry Unveiling the Secrets of Quantitative Analysis Titration In Chemistry Sentence performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. What are titrant and analyte. Learn the titration graph and titration equation. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Every titration uses the same players: The basic process involves adding a standard. Titration In Chemistry Sentence.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Titration In Chemistry Sentence Every titration uses the same players: a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What is the equivalence point of a titration. . Titration In Chemistry Sentence.
From www.youtube.com
TYPES OF TITRATION IN CHEMISTRY ACID BASE TITRATION REDOX TITRATION Titration In Chemistry Sentence a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration In Chemistry Sentence.
From worksheetcampusslewed.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration In Chemistry Sentence What is the equivalence point of a titration. Every titration uses the same players: a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution. Titration In Chemistry Sentence.
From www.scribd.com
Titration PDF Titration Chemistry Titration In Chemistry Sentence how titrations work and what you need. Every titration uses the same players: performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. What is the equivalence point of a titration. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of. Titration In Chemistry Sentence.
From resource.studiaacademy.com
Acids, Alkalis and Titrations Studia Academy Resources Titration In Chemistry Sentence how titrations work and what you need. What is the equivalence point of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of. Titration In Chemistry Sentence.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration In Chemistry Sentence a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a. Titration In Chemistry Sentence.
From mavink.com
Titration Picture Titration In Chemistry Sentence What is the equivalence point of a titration. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance. how titrations work and what you need. performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. titration is the slow. Titration In Chemistry Sentence.
From chemistnotes.com
Titration Archives Chemistry Notes Titration In Chemistry Sentence titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Every titration uses the same players: how titrations work and what you need. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is defined as ‘the. Titration In Chemistry Sentence.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration In Chemistry Sentence performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Every titration uses the same players: how titrations work and what you need. titration, process of chemical analysis. Titration In Chemistry Sentence.