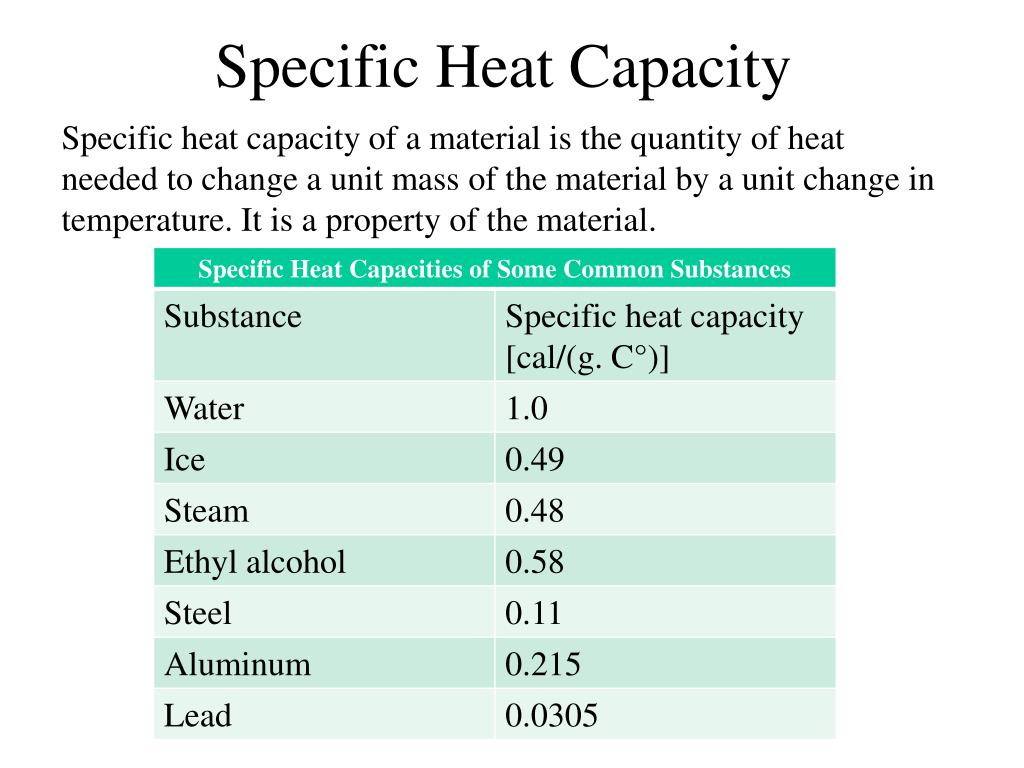
What Is Specific Heat Capacity Example . define heat capacity and specific heat capacity and differentiate between the two terms; specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have. It describes how much heat must be added to a unit of. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius.
from www.slideserve.com
define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It describes how much heat must be added to a unit of. Deduce which substance will have. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms;
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free
What Is Specific Heat Capacity Example Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms; heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). unlike the total heat capacity, the specific heat capacity is independent of mass or volume. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. It describes how much heat must be added to a unit of. Deduce which substance will have. Deduce which substance will have. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms ;
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video What Is Specific Heat Capacity Example It describes how much heat must be added to a unit of. Deduce which substance will have. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms; heat capacity. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 What Is Specific Heat Capacity Example heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. specific heat is defined. What Is Specific Heat Capacity Example.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Is Specific Heat Capacity Example heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Deduce which substance will have. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. define heat capacity and specific heat capacity and differentiate between the. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download What Is Specific Heat Capacity Example unlike the total heat capacity, the specific heat capacity is independent of mass or volume. define heat capacity and specific heat capacity and differentiate between the two terms ; the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. Deduce which substance will have.. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Heat Capacity and Specific Heat Capacity PowerPoint Presentation What Is Specific Heat Capacity Example Deduce which substance will have. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). define heat capacity and specific heat capacity and differentiate between the. What Is Specific Heat Capacity Example.
From classmediaregrouping.z14.web.core.windows.net
Calculating Specific Heat Examples What Is Specific Heat Capacity Example unlike the total heat capacity, the specific heat capacity is independent of mass or volume. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms ; define heat. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms; specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms ; unlike the total heat. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). specific heat is defined. What Is Specific Heat Capacity Example.
From www.youtube.com
Specific heat capacity YouTube What Is Specific Heat Capacity Example heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Deduce which substance will have. It describes how much heat must be added to a unit of. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one. What Is Specific Heat Capacity Example.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms; the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms ; heat capacity is the amount of. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Example specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It describes how much heat must be added to a unit of. Deduce which substance. What Is Specific Heat Capacity Example.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms ; heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Deduce which substance will have. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. the specific heat capacity. What Is Specific Heat Capacity Example.
From www.youtube.com
Finding Specific Heat Capacity IGCSE Physics YouTube What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms ; the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. It describes how much heat must be added to a unit of. define heat capacity and specific heat capacity and. What Is Specific Heat Capacity Example.
From quizzlibhofmann.z19.web.core.windows.net
Specific Heat Calculation Examples What Is Specific Heat Capacity Example the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms ; heat capacity is the amount of heat required to raise the temperature of an object. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Example Deduce which substance will have. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It describes how much heat. What Is Specific Heat Capacity Example.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is Specific Heat Capacity Example specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms ; unlike the total heat capacity, the specific heat capacity is independent of mass. What Is Specific Heat Capacity Example.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Is Specific Heat Capacity Example heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Deduce which substance will have. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of. What Is Specific Heat Capacity Example.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is Specific Heat Capacity Example Deduce which substance will have. Deduce which substance will have. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It describes how much heat must be added to a unit of. define heat. What Is Specific Heat Capacity Example.
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube What Is Specific Heat Capacity Example unlike the total heat capacity, the specific heat capacity is independent of mass or volume. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms ; define heat capacity and. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Example specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms; heat capacity is the amount of heat required to raise the temperature of an. What Is Specific Heat Capacity Example.
From courses.lumenlearning.com
Specific Heat Boundless Physics What Is Specific Heat Capacity Example It describes how much heat must be added to a unit of. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. Deduce which substance will have.. What Is Specific Heat Capacity Example.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have. Deduce which substance will have. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). the specific heat capacity of a material is the energy required to raise one kilogram. What Is Specific Heat Capacity Example.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Is Specific Heat Capacity Example Deduce which substance will have. It describes how much heat must be added to a unit of. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. define heat capacity and specific heat capacity and differentiate between the two terms ; heat capacity is the amount of heat required to raise the. What Is Specific Heat Capacity Example.
From studymind.co.uk
Heat Capacity Study Mind What Is Specific Heat Capacity Example heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). define heat capacity and specific heat capacity and differentiate between the two terms ; the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. It describes. What Is Specific Heat Capacity Example.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms; specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms ; It describes how much heat. What Is Specific Heat Capacity Example.
From www.youtube.com
Lec10 Specific Heat Capacity Example 3 YouTube What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms ; heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It describes how much heat must be added to a unit of. the specific heat capacity of a material is the energy required to raise. What Is Specific Heat Capacity Example.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms; unlike the total heat capacity, the specific heat capacity is independent of mass or volume. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Deduce which substance will have. define heat capacity and specific. What Is Specific Heat Capacity Example.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Specific Heat Capacity Example It describes how much heat must be added to a unit of. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have. unlike the total. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms; define heat capacity and specific heat capacity and differentiate between the two terms ; unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Deduce which substance will have. Deduce which substance will have. It describes how much heat. What Is Specific Heat Capacity Example.
From thechemistrynotes.com
Heat Capacity and Specific Heat What Is Specific Heat Capacity Example It describes how much heat must be added to a unit of. the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat is defined as the amount of. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms; define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have. It describes how much heat must be added to a unit of. unlike the total heat capacity, the specific heat capacity is independent of mass. What Is Specific Heat Capacity Example.
From studylib.net
1.3 Specific Heat Capacity What Is Specific Heat Capacity Example the specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Deduce which substance will have. heat capacity is the amount of heat required to raise the temperature of an. What Is Specific Heat Capacity Example.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Example It describes how much heat must be added to a unit of. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms; heat capacity. What Is Specific Heat Capacity Example.
From studylib.net
Specific Heat Capacity science What Is Specific Heat Capacity Example unlike the total heat capacity, the specific heat capacity is independent of mass or volume. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). define heat capacity and specific heat capacity and differentiate between the two terms ; the specific heat capacity of a material is the. What Is Specific Heat Capacity Example.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula What Is Specific Heat Capacity Example define heat capacity and specific heat capacity and differentiate between the two terms; Deduce which substance will have. unlike the total heat capacity, the specific heat capacity is independent of mass or volume. define heat capacity and specific heat capacity and differentiate between the two terms ; It describes how much heat must be added to a. What Is Specific Heat Capacity Example.