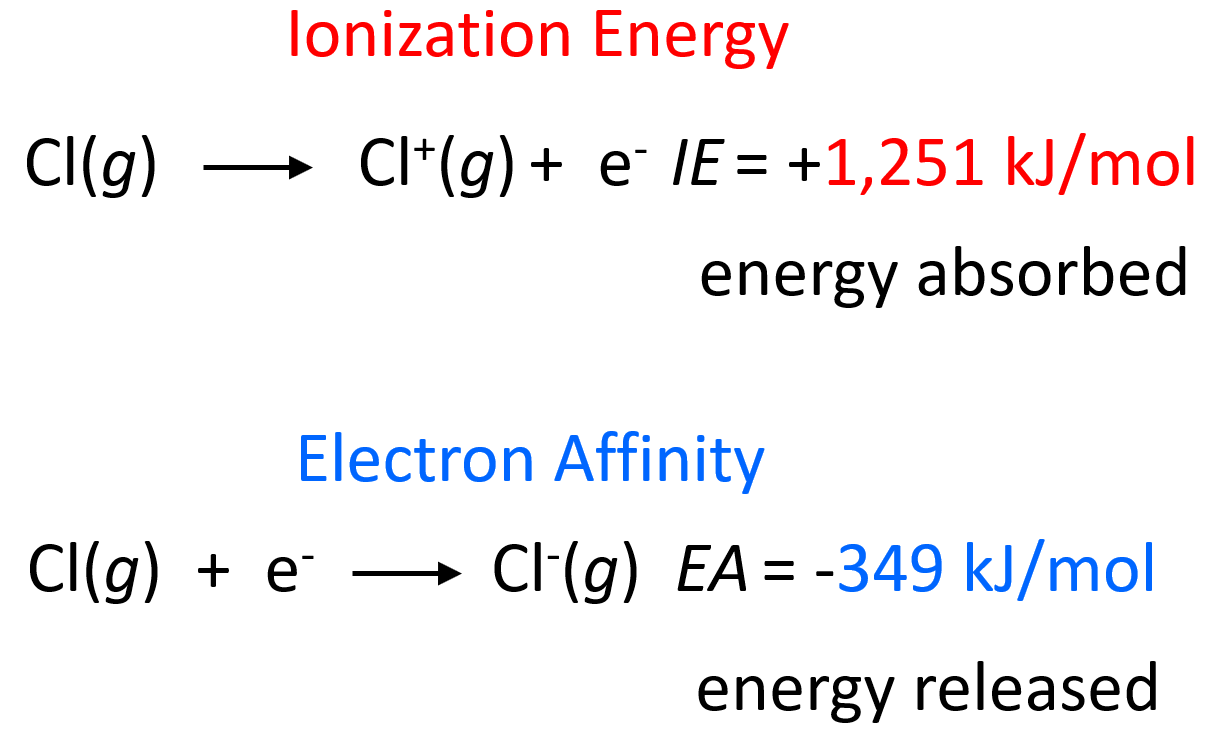
Chlorine Has More Electron Affinity Than Fluorine . Since this periodic property tends from the bottom up (in the group), the. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Why does chlorine have a higher electron affinity value than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Why does chlorine have a higher electron affinity than fluorine? Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron.
from general.chemistrysteps.com
Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Since this periodic property tends from the bottom up (in the group), the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Why does chlorine have a higher electron affinity value than fluorine? Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, therefore, has a lower affinity for an added electron than does chlorine.
Electron Affinity Chemistry Steps
Chlorine Has More Electron Affinity Than Fluorine Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Why does chlorine have a higher electron affinity than fluorine? Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Why does chlorine have a higher electron affinity value than fluorine? Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Since this periodic property tends from the bottom up (in the group), the.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Has More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Why does chlorine have a higher electron affinity than fluorine? Why does chlorine have a higher electron affinity value than fluorine? Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since chlorine's outermost orbital is a 3p. Chlorine Has More Electron Affinity Than Fluorine.
From www.youtube.com
The lower electron affinity of fluorine than that of chlorine is due to Chlorine Has More Electron Affinity Than Fluorine Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions.. Chlorine Has More Electron Affinity Than Fluorine.
From www.doubtnut.com
The lower electron affinity of fluorine than that of chlorine is due t Chlorine Has More Electron Affinity Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, therefore, has a lower affinity for an added electron than does chlorine.. Chlorine Has More Electron Affinity Than Fluorine.
From ar.inspiredpencil.com
Periodic Table With Electron Affinity Chlorine Has More Electron Affinity Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Why does chlorine have a higher electron affinity than fluorine? Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine is much more reactive than chlorine. Chlorine Has More Electron Affinity Than Fluorine.
From mavink.com
Periodic Table With Electron Affinity Chlorine Has More Electron Affinity Than Fluorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Why does chlorine have. Chlorine Has More Electron Affinity Than Fluorine.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Has More Electron Affinity Than Fluorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Why does chlorine have a higher electron affinity. Chlorine Has More Electron Affinity Than Fluorine.
From material-properties.org
Chlorine and Iodine Comparison Properties Material Properties Chlorine Has More Electron Affinity Than Fluorine Why does chlorine have a higher electron affinity value than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra. Chlorine Has More Electron Affinity Than Fluorine.
From brainly.in
Why chlorine has a higher electron affinity than fluorine? Brainly.in Chlorine Has More Electron Affinity Than Fluorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Why does chlorine have a higher electron affinity than fluorine? Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine is much more reactive than chlorine (despite the lower electron. Chlorine Has More Electron Affinity Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Chlorine Has More Electron Affinity Than Fluorine Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since this periodic property tends from the bottom up (in the group), the. Consequently, the elements of the third row ( n = 3) have the most negative. Chlorine Has More Electron Affinity Than Fluorine.
From www.toppr.com
The electron affinity of fluorine is less than that of chlorine because Chlorine Has More Electron Affinity Than Fluorine Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Why does chlorine have a higher electron affinity value than fluorine? Consequently, the elements of the third. Chlorine Has More Electron Affinity Than Fluorine.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID5631786 Chlorine Has More Electron Affinity Than Fluorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Why does chlorine have a higher electron affinity value than fluorine? Fluorine is much more reactive than chlorine (despite the lower. Chlorine Has More Electron Affinity Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Chlorine Has More Electron Affinity Than Fluorine Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since this periodic property tends from the bottom up (in. Chlorine Has More Electron Affinity Than Fluorine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Chlorine Has More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Since this periodic property tends from the bottom up (in the group), the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy. Chlorine Has More Electron Affinity Than Fluorine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Chlorine Has More Electron Affinity Than Fluorine Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Why does chlorine have a higher electron affinity value than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. Chlorine Has More Electron Affinity Than Fluorine.
From www.doubtnut.com
Chlorine has............. (more / less) electron affinity than flu Chlorine Has More Electron Affinity Than Fluorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since this periodic property tends from. Chlorine Has More Electron Affinity Than Fluorine.
From utedzz.blogspot.com
Periodic Table Fluorine Valence Electrons Periodic Table Timeline Chlorine Has More Electron Affinity Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Consequently, the elements of the third row ( n =. Chlorine Has More Electron Affinity Than Fluorine.
From www.nagwa.com
Question Video Identifying the Explanation for the Anomalous Electron Chlorine Has More Electron Affinity Than Fluorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Why does chlorine have a higher electron affinity value than fluorine? Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine, therefore, has a lower affinity for an added electron. Chlorine Has More Electron Affinity Than Fluorine.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Has More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Why does chlorine have a higher electron affinity value than fluorine? Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Why does chlorine have a higher electron affinity than fluorine? Since this periodic property tends from the bottom up. Chlorine Has More Electron Affinity Than Fluorine.
From exosuznkq.blob.core.windows.net
Chlorine Has A Greater Electron Affinity Than Sodium at Janice Bourn blog Chlorine Has More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron.. Chlorine Has More Electron Affinity Than Fluorine.
From www.doubtnut.com
Electron affinity of chlorine is larger than that of fluorine because Chlorine Has More Electron Affinity Than Fluorine Since this periodic property tends from the bottom up (in the group), the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Why does chlorine have a higher electron affinity value than. Chlorine Has More Electron Affinity Than Fluorine.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Chlorine Has More Electron Affinity Than Fluorine Since this periodic property tends from the bottom up (in the group), the. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since chlorine's outermost orbital is a 3p orbital, there is more space,. Chlorine Has More Electron Affinity Than Fluorine.
From www.nagwa.com
Question Video Determining the Equation that Represents the Electron Chlorine Has More Electron Affinity Than Fluorine Why does chlorine have a higher electron affinity than fluorine? Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Why does chlorine have a higher electron affinity value than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions.. Chlorine Has More Electron Affinity Than Fluorine.
From questions-in.kunduz.com
13. . The electron affinity of fluorine i... Physical Chemistry Chlorine Has More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to. Chlorine Has More Electron Affinity Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Chlorine Has More Electron Affinity Than Fluorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Why does chlorine have. Chlorine Has More Electron Affinity Than Fluorine.
From www.collegesearch.in
Electron Affinity Factors Affecting, Halogens, Periodic Trends, Metals Chlorine Has More Electron Affinity Than Fluorine Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Since this periodic property tends from the bottom up (in the group), the. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Consequently, the elements of the third row ( n = 3) have. Chlorine Has More Electron Affinity Than Fluorine.
From ar.inspiredpencil.com
Electron Affinity List Chlorine Has More Electron Affinity Than Fluorine Since this periodic property tends from the bottom up (in the group), the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps. Chlorine Has More Electron Affinity Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Chlorine Has More Electron Affinity Than Fluorine Since this periodic property tends from the bottom up (in the group), the. Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons. Chlorine Has More Electron Affinity Than Fluorine.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Chlorine Has More Electron Affinity Than Fluorine Since this periodic property tends from the bottom up (in the group), the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Since chlorine's. Chlorine Has More Electron Affinity Than Fluorine.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Chlorine Has More Electron Affinity Than Fluorine Why does chlorine have a higher electron affinity than fluorine? Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in. Chlorine Has More Electron Affinity Than Fluorine.
From www.toppr.com
Electron affinity of chlorine is more than that of fluorine though Chlorine Has More Electron Affinity Than Fluorine Why does chlorine have a higher electron affinity value than fluorine? Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Since this periodic property tends from the bottom up (in the group), the. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Why does chlorine have a higher electron affinity. Chlorine Has More Electron Affinity Than Fluorine.
From www.doubtnut.com
Electron affinity of chlorine is larger than that of fluorine because Chlorine Has More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since chlorine's outermost orbital is a. Chlorine Has More Electron Affinity Than Fluorine.
From www.youtube.com
Why is electron affinity of chlorine more than thatof fluorine? CLASS Chlorine Has More Electron Affinity Than Fluorine Why does chlorine have a higher electron affinity than fluorine? Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since this periodic property tends from the bottom up (in the group), the. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy. Chlorine Has More Electron Affinity Than Fluorine.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Has More Electron Affinity Than Fluorine Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy. Chlorine Has More Electron Affinity Than Fluorine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Chlorine Has More Electron Affinity Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since this periodic property tends from the bottom up (in the group), the. Why does chlorine have a higher electron affinity than fluorine? Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share. Chlorine Has More Electron Affinity Than Fluorine.
From www.toppr.com
viii. Crystal radius is larger than covalent radius. ix. Electron Chlorine Has More Electron Affinity Than Fluorine Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Since this periodic property tends from the bottom up (in the group), the. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions. Fluorine, though higher. Chlorine Has More Electron Affinity Than Fluorine.