Negative Electrode Definition In Chemistry . From the reaction perspective, as the reductant. Generally, the cathode is the. In a lithium ion cell the anode is. Excess positive ions at the cathode are repelled to the anode but move more slowly. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. Ionic compound in molten or dissolved solution that conducts the electricity. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. Thus, when δg is negative the reaction is. This can be understood from two perspectives. Cations are attracted to the cathode. In a galvanic cell this is the negative electrode. In an electrochemical cell, the cathode is the electrode at which reduction occurs. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. The positive electrode of an electrolysis cell. The negative electrode of the external voltage source supplies electrons to the anode.
from www.snexplores.org
For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. From the reaction perspective, as the reductant. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Ionic compound in molten or dissolved solution that conducts the electricity. In a galvanic cell this is the negative electrode. Cations are attracted to the cathode. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. Excess positive ions at the cathode are repelled to the anode but move more slowly. Generally, the cathode is the. The negative electrode of the external voltage source supplies electrons to the anode.
Explainer What is an electrode?
Negative Electrode Definition In Chemistry Excess positive ions at the cathode are repelled to the anode but move more slowly. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. Generally, the cathode is the. From the reaction perspective, as the reductant. Ionic compound in molten or dissolved solution that conducts the electricity. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Thus, when δg is negative the reaction is. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. In an electrochemical cell, the cathode is the electrode at which reduction occurs. Cations are attracted to the cathode. Excess positive ions at the cathode are repelled to the anode but move more slowly. In a lithium ion cell the anode is. In a galvanic cell this is the negative electrode. This can be understood from two perspectives. The positive electrode of an electrolysis cell.
From resource.studiaacademy.com
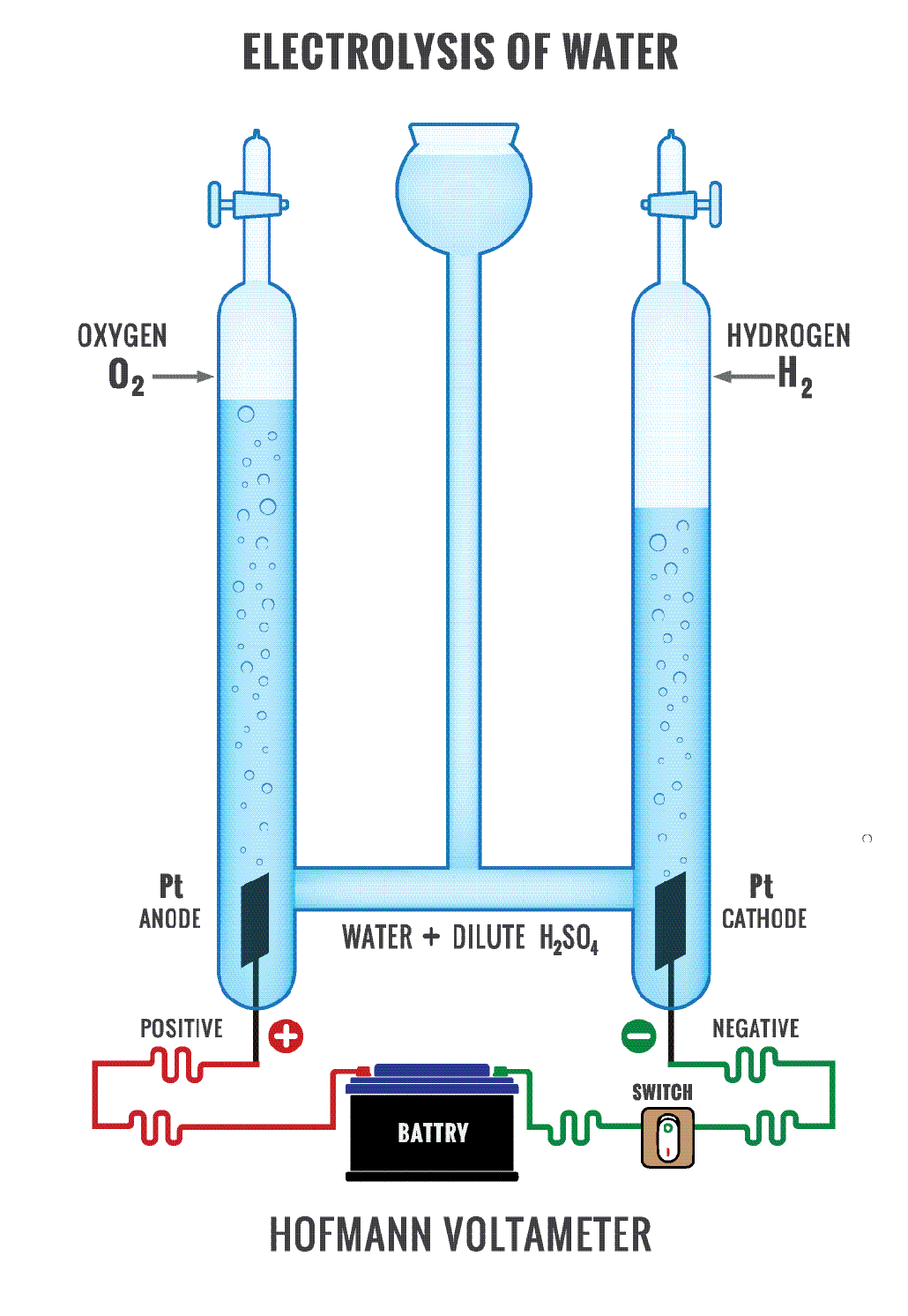
1.9 Electrolysis Studia Academy Resources Negative Electrode Definition In Chemistry The positive electrode of an electrolysis cell. Thus, when δg is negative the reaction is. In a galvanic cell this is the negative electrode. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. The negative electrode of the external voltage source supplies electrons to the anode.. Negative Electrode Definition In Chemistry.
From mungfali.com
Reference Electrode Diagram Negative Electrode Definition In Chemistry Excess positive ions at the cathode are repelled to the anode but move more slowly. This can be understood from two perspectives. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. Thus, when δg is negative the reaction is. Generally, the cathode is the. The electrode attached to the negative terminal of. Negative Electrode Definition In Chemistry.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Negative Electrode Definition In Chemistry From the reaction perspective, as the reductant. Thus, when δg is negative the reaction is. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. Ionic compound in molten or dissolved solution that conducts the electricity. Generally, the cathode is the. In a lithium ion cell the anode is.. Negative Electrode Definition In Chemistry.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Negative Electrode Definition In Chemistry In an electrochemical cell, the cathode is the electrode at which reduction occurs. Generally, the cathode is the. The negative electrode of the external voltage source supplies electrons to the anode. Cations are attracted to the cathode. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. This can be understood from two. Negative Electrode Definition In Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Negative Electrode Definition In Chemistry The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Thus, when δg is negative the reaction is. In an electrochemical cell, the cathode is the electrode at which reduction occurs. Generally, the cathode is the. In a galvanic cell this is the negative electrode. Excess positive ions at the cathode are repelled. Negative Electrode Definition In Chemistry.
From www.slideserve.com
PPT Anode PowerPoint Presentation, free download ID3222462 Negative Electrode Definition In Chemistry The positive electrode of an electrolysis cell. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. The negative electrode of the external voltage source supplies electrons to the anode. In an electrochemical cell, the cathode is the electrode at which reduction occurs. Excess positive ions at. Negative Electrode Definition In Chemistry.
From www.slideserve.com
PPT Electrolysis of Solutions PowerPoint Presentation, free download Negative Electrode Definition In Chemistry In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. From the reaction perspective, as the reductant. In a galvanic cell this is the negative electrode. In an electrochemical cell,. Negative Electrode Definition In Chemistry.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Negative Electrode Definition In Chemistry Cations are attracted to the cathode. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. In a galvanic cell this is the negative electrode. Generally, the cathode is the. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. In a. Negative Electrode Definition In Chemistry.
From byjus.com
An electrolytic cell is used to convert Negative Electrode Definition In Chemistry In an electrochemical cell, the cathode is the electrode at which reduction occurs. This can be understood from two perspectives. From the reaction perspective, as the reductant. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. In a lithium ion cell the anode is. In a galvanic cell this is the negative. Negative Electrode Definition In Chemistry.
From mavink.com
Electrode Classification Diagram Negative Electrode Definition In Chemistry Thus, when δg is negative the reaction is. In a lithium ion cell the anode is. The positive electrode of an electrolysis cell. From the reaction perspective, as the reductant. Excess positive ions at the cathode are repelled to the anode but move more slowly. This can be understood from two perspectives. Generally, the cathode is the. Ionic compound in. Negative Electrode Definition In Chemistry.
From www.researchgate.net
Negative electrode chemistry for pure silicon and Sibased materials. A Negative Electrode Definition In Chemistry In an electrochemical cell, the cathode is the electrode at which reduction occurs. Thus, when δg is negative the reaction is. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. In a galvanic cell this is the negative electrode. The positive electrode of an electrolysis cell. For a spontaneous reaction, e cell. Negative Electrode Definition In Chemistry.
From www.researchgate.net
Schematic of the (negative) electrodeelectrolyte interface in EDLCs Negative Electrode Definition In Chemistry For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when. Negative Electrode Definition In Chemistry.
From 2012books.lardbucket.org
Standard Potentials Negative Electrode Definition In Chemistry The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Generally, the cathode is the. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. The positive electrode of an electrolysis cell. In a galvanic cell this is the negative. Negative Electrode Definition In Chemistry.
From www.researchgate.net
XRD patterns of the bCo(OH) 2 electrode at the charge and discharge Negative Electrode Definition In Chemistry For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. Cations are attracted to the cathode. Excess positive ions at the cathode are repelled to the anode but move more slowly. Generally, the cathode is the. In an electrochemical cell, the cathode is the electrode at which. Negative Electrode Definition In Chemistry.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode Negative Electrode Definition In Chemistry The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. In an electrochemical cell, the cathode is the electrode at which reduction occurs. In a galvanic cell this is the negative electrode. The negative electrode of the external voltage source supplies electrons to the anode. In a galvanic cell,. Negative Electrode Definition In Chemistry.
From byjus.com
What is the negative electrode called in electrolysis? Negative Electrode Definition In Chemistry The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. Ionic compound in molten or dissolved solution that conducts the electricity. In a galvanic cell, the reaction is spontaneous, there. Negative Electrode Definition In Chemistry.
From www.theknowledgelibrary.in
Difference Between Cation & Anion The Knowledge Library Negative Electrode Definition In Chemistry In an electrochemical cell, the cathode is the electrode at which reduction occurs. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. This can be understood from two perspectives. The negative electrode of the external voltage source supplies electrons to the anode. From the reaction perspective, as the reductant. The anode is. Negative Electrode Definition In Chemistry.
From saylordotorg.github.io
Electrochemistry Negative Electrode Definition In Chemistry The positive electrode of an electrolysis cell. In a galvanic cell this is the negative electrode. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. From the reaction perspective, as the reductant. Ionic compound in molten or dissolved solution that conducts the electricity. The electrode attached. Negative Electrode Definition In Chemistry.
From www.thoughtco.com
How to Define Anode and Cathode Negative Electrode Definition In Chemistry For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. From the reaction perspective, as the reductant. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. The negative electrode of the external voltage source supplies electrons to the anode.. Negative Electrode Definition In Chemistry.
From mathformula5.netlify.app
What Is The Definition Of Formula Unit In Chemistry Complete Guide Negative Electrode Definition In Chemistry In a lithium ion cell the anode is. Ionic compound in molten or dissolved solution that conducts the electricity. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. Thus, when δg is negative the reaction is. The positive electrode of an electrolysis cell. In a galvanic. Negative Electrode Definition In Chemistry.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material Negative Electrode Definition In Chemistry Excess positive ions at the cathode are repelled to the anode but move more slowly. In a galvanic cell this is the negative electrode. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The negative electrode of the external voltage source supplies electrons to the anode. In a galvanic cell, the reaction. Negative Electrode Definition In Chemistry.
From saylordotorg.github.io
Standard Potentials Negative Electrode Definition In Chemistry In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. The negative electrode of the external voltage source supplies electrons to the anode. Ionic compound in molten or dissolved solution that conducts the electricity. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and. Negative Electrode Definition In Chemistry.
From www.alamy.com
Ions movement to negative electrode and positive electrode. 3D Negative Electrode Definition In Chemistry In a lithium ion cell the anode is. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. From the reaction perspective, as the reductant. Ionic compound in molten or dissolved solution that conducts the electricity. This can be understood from two perspectives. Generally, the cathode is the. In. Negative Electrode Definition In Chemistry.
From stock.adobe.com
Ions movement to negative electrode and positive electrode Stock Photo Negative Electrode Definition In Chemistry For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. The negative electrode of the external voltage source supplies electrons to the anode. Ionic compound in molten or dissolved solution that conducts the electricity. This can be understood from two perspectives. In a galvanic cell this is. Negative Electrode Definition In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Negative Electrode Definition In Chemistry In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. The positive electrode of an electrolysis cell. Excess positive ions at the cathode are repelled to the anode but move more slowly. This can be understood from two perspectives. In a lithium ion cell the anode is. The negative electrode of the external. Negative Electrode Definition In Chemistry.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Negative Electrode Definition In Chemistry For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. In an electrochemical cell, the cathode is the electrode at which reduction occurs. Thus, when δg is negative the reaction is. In a lithium ion cell the anode is. The electrode attached to the negative terminal of. Negative Electrode Definition In Chemistry.
From www.snexplores.org
Explainer What is an electrode? Negative Electrode Definition In Chemistry Ionic compound in molten or dissolved solution that conducts the electricity. In a galvanic cell this is the negative electrode. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. This can be understood from two perspectives. The anode is the negative or reducing electrode that releases. Negative Electrode Definition In Chemistry.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Negative Electrode Definition In Chemistry Thus, when δg is negative the reaction is. Generally, the cathode is the. In a lithium ion cell the anode is. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. In an electrochemical cell, the cathode is the electrode at which reduction occurs. The electrode attached. Negative Electrode Definition In Chemistry.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? Negative Electrode Definition In Chemistry In a galvanic cell this is the negative electrode. In an electrochemical cell, the cathode is the electrode at which reduction occurs. The positive electrode of an electrolysis cell. Ionic compound in molten or dissolved solution that conducts the electricity. This can be understood from two perspectives. The anode is the negative or reducing electrode that releases electrons to the. Negative Electrode Definition In Chemistry.
From www.chemistry-teaching-resources.com
chemistry picture Negative Electrode Definition In Chemistry Ionic compound in molten or dissolved solution that conducts the electricity. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. Generally, the cathode is the. The positive electrode of an electrolysis cell. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. From the reaction. Negative Electrode Definition In Chemistry.
From question.pandai.org
Standard Electrode Potential Negative Electrode Definition In Chemistry Generally, the cathode is the. In a lithium ion cell the anode is. In a galvanic cell, the reaction is spontaneous, there is no external potential applied, and when the. In a galvanic cell this is the negative electrode. Thus, when δg is negative the reaction is. The electrode attached to the negative terminal of a battery is called a. Negative Electrode Definition In Chemistry.
From study.com
Electrode Definition, Types & Function Lesson Negative Electrode Definition In Chemistry Generally, the cathode is the. From the reaction perspective, as the reductant. Excess positive ions at the cathode are repelled to the anode but move more slowly. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The positive electrode of an electrolysis cell. This can be understood from two perspectives. In a. Negative Electrode Definition In Chemistry.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Negative Electrode Definition In Chemistry Ionic compound in molten or dissolved solution that conducts the electricity. This can be understood from two perspectives. The negative electrode of the external voltage source supplies electrons to the anode. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. In a lithium ion cell the. Negative Electrode Definition In Chemistry.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube Negative Electrode Definition In Chemistry The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. In an electrochemical cell, the cathode is the electrode at which reduction occurs. For a spontaneous reaction, e cell is positive and δg (gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. In a lithium. Negative Electrode Definition In Chemistry.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Negative Electrode Definition In Chemistry The negative electrode of the external voltage source supplies electrons to the anode. Thus, when δg is negative the reaction is. Generally, the cathode is the. From the reaction perspective, as the reductant. In an electrochemical cell, the cathode is the electrode at which reduction occurs. In a lithium ion cell the anode is. Excess positive ions at the cathode. Negative Electrode Definition In Chemistry.