Zinc And Copper Are Examples Of . A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Updated on september 14, 2024. The proportions of the copper and zinc are varied to. Proposed fortification levels and recommended zinc compounds. “zinc and copper is an example of a solution of “an alloy through which brass is formed. Brass is an alloy made primarily of copper and zinc. While both metals have similar properties, there are some key differences that could make all the. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. Are you trying to decide between zinc and copper for your next project? The alloy may be considered a solid solution or a. When a sheet of zinc is placed in an aqueous.
from www.numerade.com
A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The proportions of the copper and zinc are varied to. Brass is an alloy made primarily of copper and zinc. In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. The alloy may be considered a solid solution or a. “zinc and copper is an example of a solution of “an alloy through which brass is formed. While both metals have similar properties, there are some key differences that could make all the. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. Updated on september 14, 2024. When a sheet of zinc is placed in an aqueous.
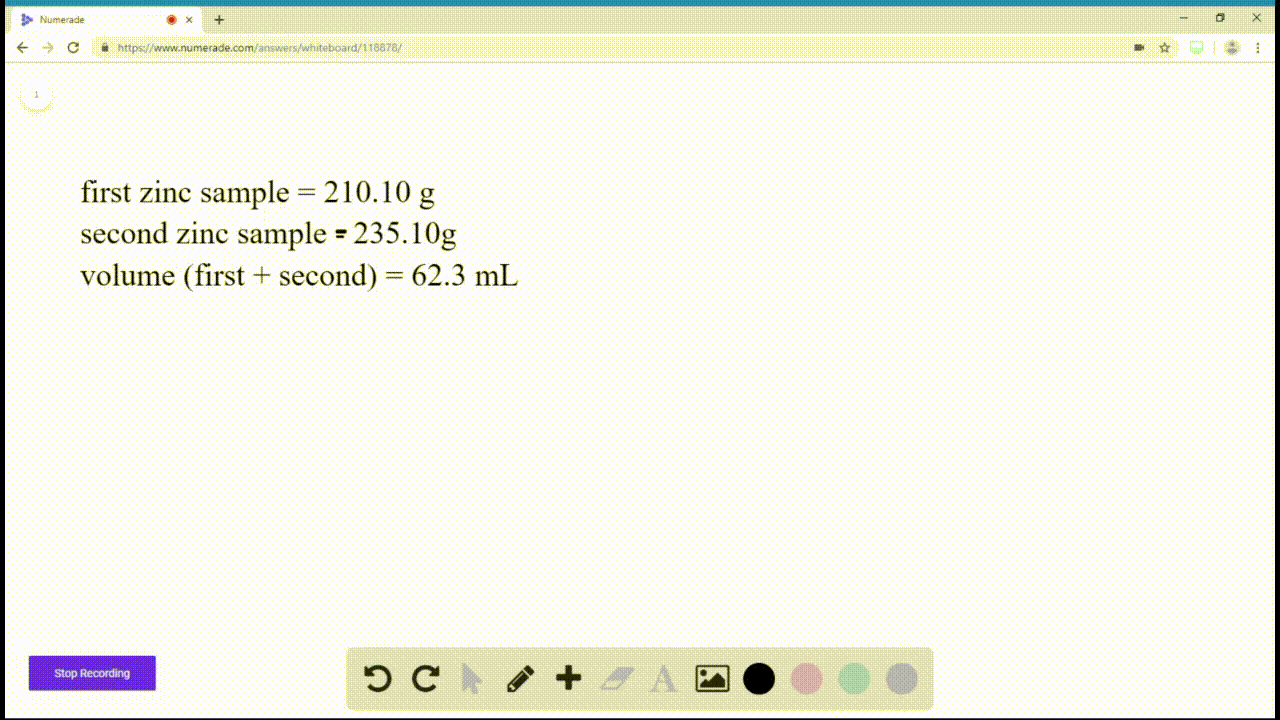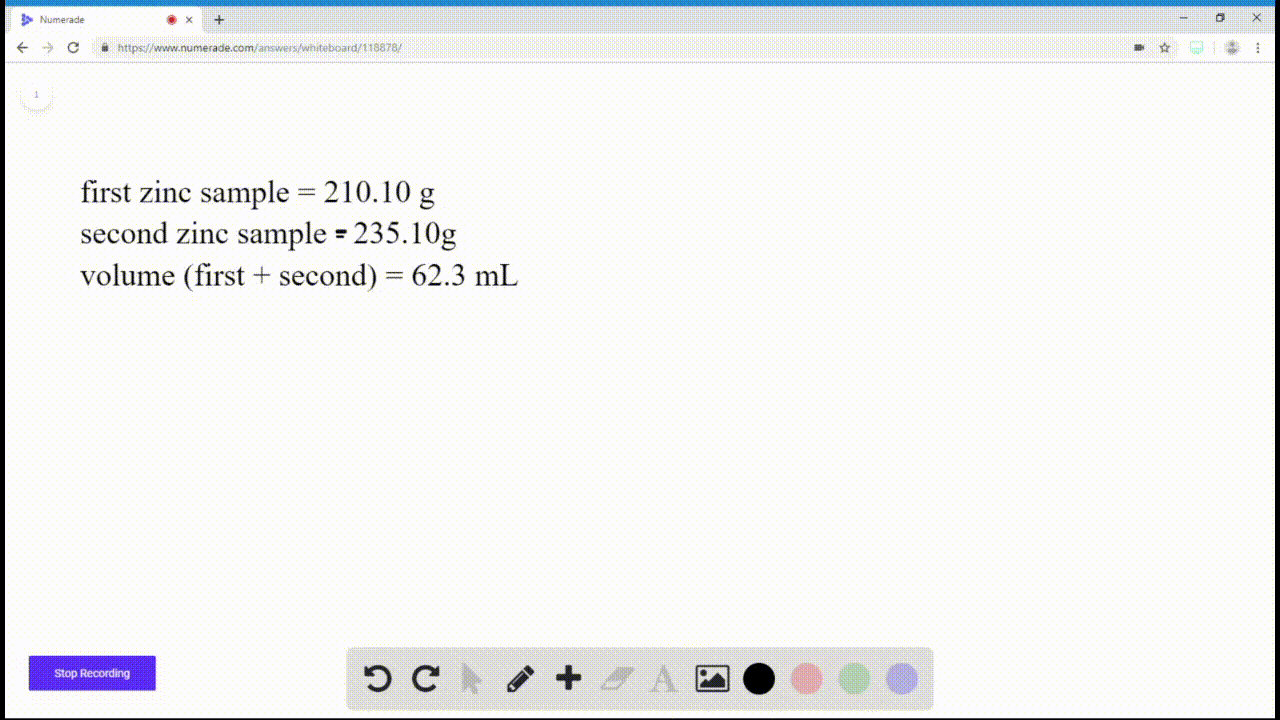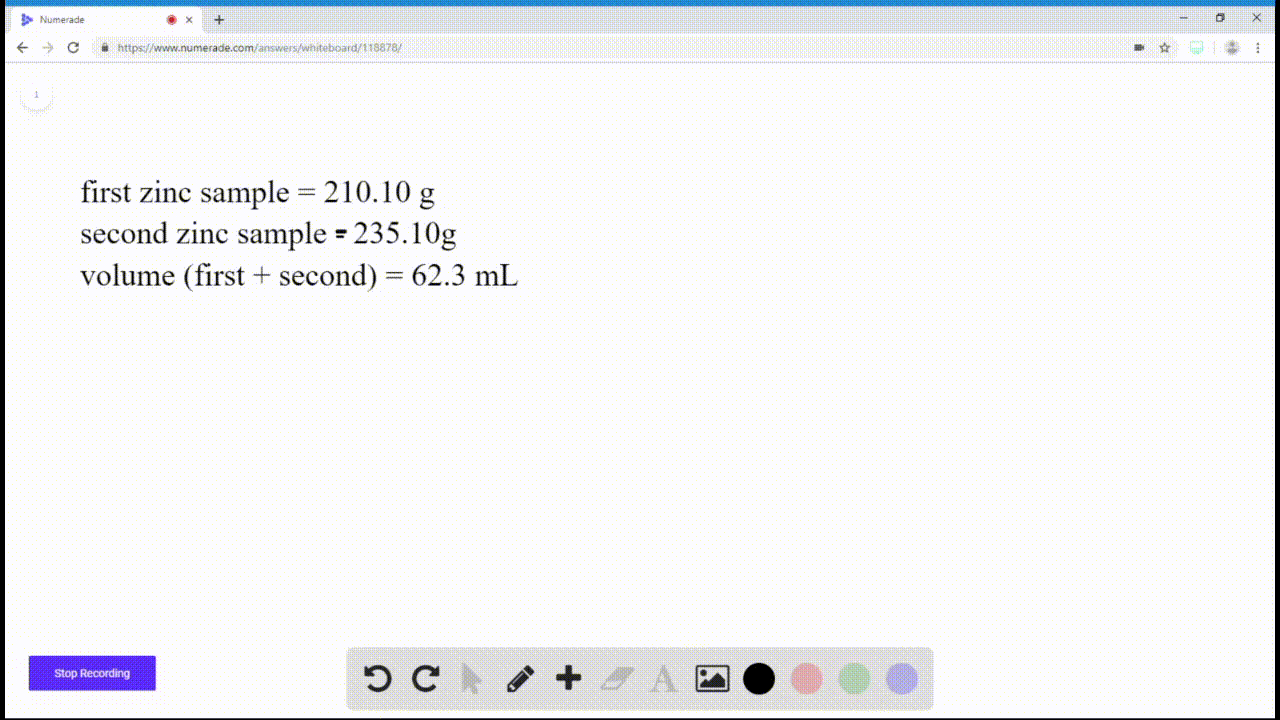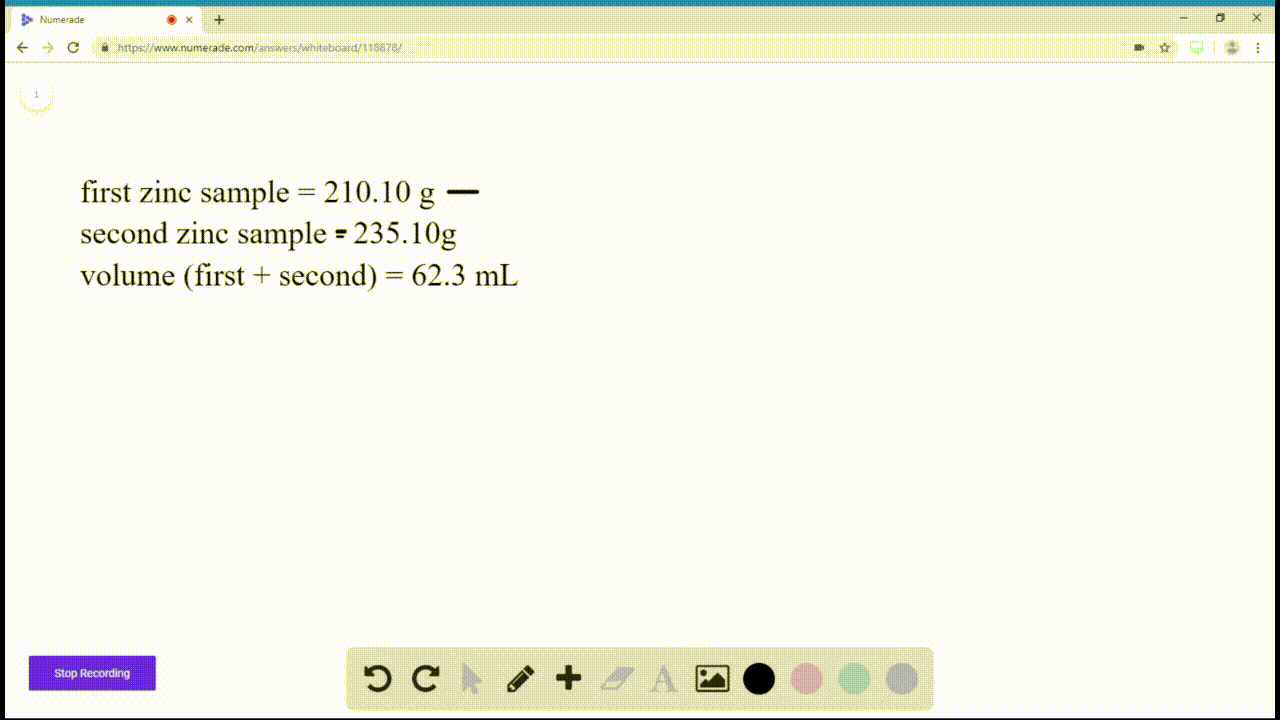
SOLVEDIf you have a sample of zinc and a sample of copper, and both
Zinc And Copper Are Examples Of When a sheet of zinc is placed in an aqueous. In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Updated on september 14, 2024. Brass is an alloy made primarily of copper and zinc. While both metals have similar properties, there are some key differences that could make all the. Proposed fortification levels and recommended zinc compounds. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. When a sheet of zinc is placed in an aqueous. Are you trying to decide between zinc and copper for your next project? “zinc and copper is an example of a solution of “an alloy through which brass is formed. The proportions of the copper and zinc are varied to. The alloy may be considered a solid solution or a. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Zinc And Copper Are Examples Of In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Brass is an alloy made primarily of copper and zinc. “zinc and copper is an example of a solution of “an alloy through which brass is formed. Updated on september 14, 2024. When a sheet of. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Zinc vs. Copper What's the Difference Zinc And Copper Are Examples Of Brass is an alloy made primarily of copper and zinc. “zinc and copper is an example of a solution of “an alloy through which brass is formed. Proposed fortification levels and recommended zinc compounds. Updated on september 14, 2024. When a sheet of zinc is placed in an aqueous. Iron (fe), copper (cu), and zinc (zn) are elements on which. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Copper Zinc Alloys Properties and Uses Zinc And Copper Are Examples Of In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. “zinc and copper is an example of a solution of “an alloy through which brass is formed. Are you trying to decide between zinc and copper for your next project? Updated on september 14, 2024. When. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
An Introduction to CopperZinc Alloys Zinc And Copper Are Examples Of When a sheet of zinc is placed in an aqueous. Are you trying to decide between zinc and copper for your next project? Brass is an alloy made primarily of copper and zinc. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. A typical cell might consist. Zinc And Copper Are Examples Of.
From periodictable.com
Pre1982 Pennies, a sample of the element Copper in the Periodic Table Zinc And Copper Are Examples Of Are you trying to decide between zinc and copper for your next project? In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Updated on september 14, 2024. When a sheet of zinc is placed in an aqueous. While both metals have similar properties, there are. Zinc And Copper Are Examples Of.
From fyoowqcvt.blob.core.windows.net
Zinc And Copper Nitrate at Crystle Johnson blog Zinc And Copper Are Examples Of When a sheet of zinc is placed in an aqueous. The alloy may be considered a solid solution or a. Brass is an alloy made primarily of copper and zinc. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. Updated on september 14, 2024. The proportions of. Zinc And Copper Are Examples Of.
From theodoregray.com
Sample of the element Copper in the Periodic Table Zinc And Copper Are Examples Of Updated on september 14, 2024. Brass is an alloy made primarily of copper and zinc. In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
A Guide to Common Metals Iron, Copper, and Zinc Zinc And Copper Are Examples Of “zinc and copper is an example of a solution of “an alloy through which brass is formed. Proposed fortification levels and recommended zinc compounds. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. In grade 11, you carried out an experiment to see what happens when zinc. Zinc And Copper Are Examples Of.
From warpiano.blogspot.com
Foods With Lots Of Zinc Food Network B Zinc And Copper Are Examples Of “zinc and copper is an example of a solution of “an alloy through which brass is formed. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The proportions of the copper and zinc are varied to. Iron (fe), copper. Zinc And Copper Are Examples Of.
From drjockers.com
How To Test Zinc Levels At Home Zinc And Copper Are Examples Of “zinc and copper is an example of a solution of “an alloy through which brass is formed. When a sheet of zinc is placed in an aqueous. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The proportions of. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
A Breakdown of Copper vs. Zinc Reactivity Zinc And Copper Are Examples Of When a sheet of zinc is placed in an aqueous. In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Updated on september 14, 2024. Brass is an alloy made primarily of copper and zinc. “zinc and copper is an example of a solution of “an. Zinc And Copper Are Examples Of.
From www.thefoodstatecompany.com
Natural Zinc & Copper Supplement The Foodstate Company Zinc And Copper Are Examples Of Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. The proportions of the copper and zinc are varied to. While both metals have similar properties, there are some key differences that could make all the. In grade 11, you carried out an experiment to see what happens. Zinc And Copper Are Examples Of.
From www.youtube.com
An alloy of copper and zinc contains 45 copper; the rest is zinc. Find Zinc And Copper Are Examples Of A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Brass is an alloy made primarily of copper and zinc. Are you trying to decide between zinc and copper for your next project? The proportions of the copper and zinc. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Difference Between Copper and Zinc Zinc And Copper Are Examples Of Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. While both metals have similar properties, there are. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Metallic Bonding in Zinc An Overview Zinc And Copper Are Examples Of Updated on september 14, 2024. Are you trying to decide between zinc and copper for your next project? In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. The alloy may be considered a solid solution or a. “zinc and copper is an example of a. Zinc And Copper Are Examples Of.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Are Examples Of Brass is an alloy made primarily of copper and zinc. Are you trying to decide between zinc and copper for your next project? While both metals have similar properties, there are some key differences that could make all the. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Comparing the Reactivity of Copper vs Zinc Zinc And Copper Are Examples Of In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Proposed fortification levels and recommended zinc compounds. The alloy may be considered a solid solution or a. Are you trying to decide between zinc and copper for your next project? Brass is an alloy made primarily. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
What is the Ratio of Copper and Zinc in an Alloy? Zinc And Copper Are Examples Of Brass is an alloy made primarily of copper and zinc. Are you trying to decide between zinc and copper for your next project? In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. When a sheet of zinc is placed in an aqueous. Updated on september. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Advantages and Disadvantages of Copper Zinc Alloy Zinc And Copper Are Examples Of When a sheet of zinc is placed in an aqueous. Proposed fortification levels and recommended zinc compounds. Are you trying to decide between zinc and copper for your next project? Brass is an alloy made primarily of copper and zinc. “zinc and copper is an example of a solution of “an alloy through which brass is formed. Iron (fe), copper. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Metallic Oxides of Zinc, Magnesium, and Copper Zinc And Copper Are Examples Of Brass is an alloy made primarily of copper and zinc. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. Zinc And Copper Are Examples Of.
From www.cruxinvestor.com
Metal Mania Reuters on Copper, Tin, Zinc, Lithium, Cobalt and More Zinc And Copper Are Examples Of Updated on september 14, 2024. Brass is an alloy made primarily of copper and zinc. The proportions of the copper and zinc are varied to. In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. The alloy may be considered a solid solution or a. A. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Zinc And Copper Are Examples Of A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. “zinc and copper is an example of a solution of “an alloy through which brass is formed. While both metals have similar properties, there are some key differences that could. Zinc And Copper Are Examples Of.
From saylordotorg.github.io
Electrochemistry Zinc And Copper Are Examples Of A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Are you trying to decide between zinc and copper for your next project? Updated on september 14, 2024. In grade 11, you carried out an experiment to see what happens. Zinc And Copper Are Examples Of.
From www.youtube.com
Q64 The ratio of copper and zinc in brass is 137. How much zinc will Zinc And Copper Are Examples Of A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Proposed fortification levels and recommended zinc compounds. Brass is an alloy made primarily of copper and zinc. “zinc and copper is an example of a solution of “an alloy through. Zinc And Copper Are Examples Of.
From www.numerade.com
SOLVEDIf you have a sample of zinc and a sample of copper, and both Zinc And Copper Are Examples Of A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. When a sheet of zinc is placed in an aqueous. The alloy may be considered a solid solution or a. In grade 11, you carried out an experiment to see. Zinc And Copper Are Examples Of.
From www.htfmarketintelligence.com
Lead and Zinc Market Growing at Robust Expansion of the Decade Zinc And Copper Are Examples Of In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. While both metals have similar properties, there are some key differences that could make all the. Brass is an alloy made primarily of copper and zinc. “zinc and copper is an example of a solution of. Zinc And Copper Are Examples Of.
From www.hiscoll.com
Hiscoll Military Antiques Commemorative, coppertoned and zincbased Zinc And Copper Are Examples Of Are you trying to decide between zinc and copper for your next project? The alloy may be considered a solid solution or a. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. When a sheet of zinc is placed in an aqueous. A typical cell might consist. Zinc And Copper Are Examples Of.
From zinc.ca
Zinc for Copper Overload Zinc.ca Zinc And Copper Are Examples Of Are you trying to decide between zinc and copper for your next project? Brass is an alloy made primarily of copper and zinc. The alloy may be considered a solid solution or a. When a sheet of zinc is placed in an aqueous. A typical cell might consist of two pieces of metal, one zinc and the other copper, each. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Can You Copper Plate Zinc? Zinc And Copper Are Examples Of “zinc and copper is an example of a solution of “an alloy through which brass is formed. Are you trying to decide between zinc and copper for your next project? While both metals have similar properties, there are some key differences that could make all the. In grade 11, you carried out an experiment to see what happens when zinc. Zinc And Copper Are Examples Of.
From www.researchgate.net
Preparing of zinc plates and copper plates for use in the production of Zinc And Copper Are Examples Of The alloy may be considered a solid solution or a. The proportions of the copper and zinc are varied to. Brass is an alloy made primarily of copper and zinc. While both metals have similar properties, there are some key differences that could make all the. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
CopperZinc Alloys A Complete Guide Zinc And Copper Are Examples Of Brass is an alloy made primarily of copper and zinc. While both metals have similar properties, there are some key differences that could make all the. Updated on september 14, 2024. The proportions of the copper and zinc are varied to. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
Can You Take Zinc and Copper Together? Zinc And Copper Are Examples Of Brass is an alloy made primarily of copper and zinc. Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. Proposed fortification levels and recommended zinc compounds. While both metals have similar properties, there are some key differences that could make all the. Are you trying to decide. Zinc And Copper Are Examples Of.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc And Copper Are Examples Of Brass is an alloy made primarily of copper and zinc. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Are you trying to decide between zinc and copper for your next project? In grade 11, you carried out an. Zinc And Copper Are Examples Of.
From blog.thepipingmart.com
CopperZinc Alloys An Overview Zinc And Copper Are Examples Of Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. While both metals have similar properties, there are some key differences that could make all the. The proportions of the copper and zinc are varied to. Updated on september 14, 2024. “zinc and copper is an example of. Zinc And Copper Are Examples Of.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Zinc And Copper Are Examples Of Iron (fe), copper (cu), and zinc (zn) are elements on which organisms, ranging from simple bacteria all the way to complex ones. Proposed fortification levels and recommended zinc compounds. In grade 11, you carried out an experiment to see what happens when zinc granules are added to a solution of copper (ii) sulfate. Brass is an alloy made primarily of. Zinc And Copper Are Examples Of.