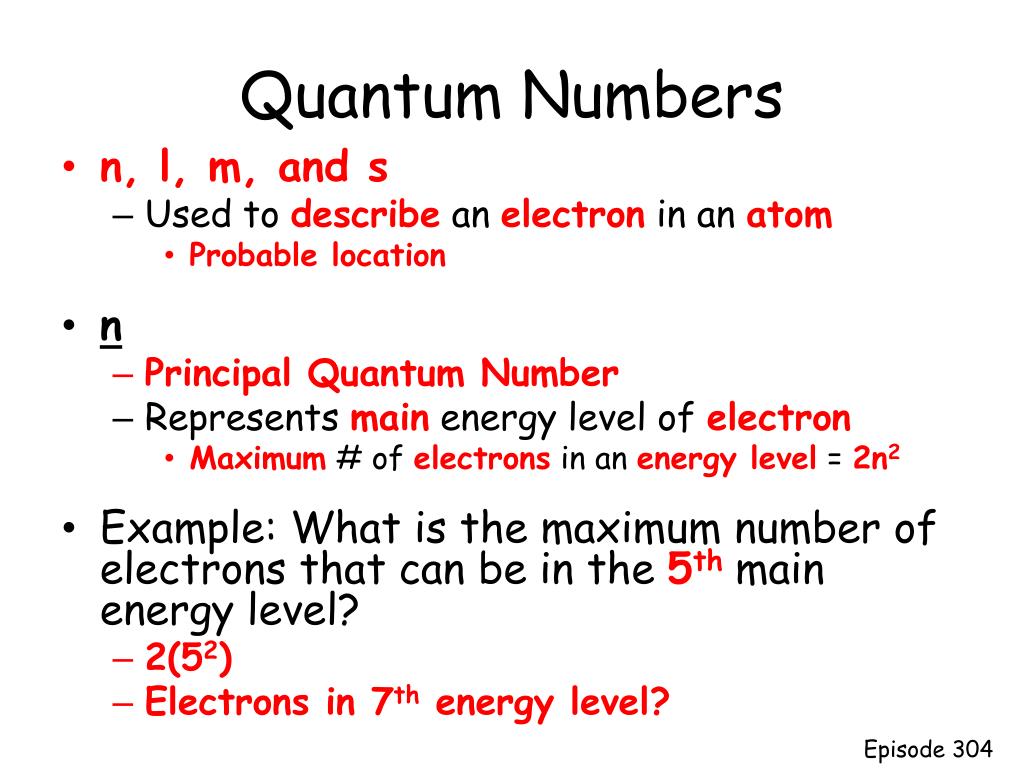
What Is Quantum Numbers And Orbitals . Find out how to combine quantum numbers and form shells and subshells of. Principal quantum number, denoted by n. Principal, angular, magnetic and spin. Find out the allowed values of n, l, and. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: Find out the allowed values, symbols and examples of each. Magnetic quantum number, denoted by m l. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. The electron spin quantum number, denoted by m s. Find out how they determine the energy, shape, orientation, and spin of orbitals. Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Explore the concepts of principal,. Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms.
from www.slideserve.com
Find out how to combine quantum numbers and form shells and subshells of. Principal, angular, magnetic and spin. Find out the allowed values of n, l, and. There is 1 s orbital and 3 p orbitals, so there are. Explore the concepts of principal,. Learn how to describe the properties of electrons in atoms using four quantum numbers: Principal quantum number, denoted by n. Find out how they determine the energy, shape, orientation, and spin of orbitals. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. The electron spin quantum number, denoted by m s.
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772
What Is Quantum Numbers And Orbitals Find out the allowed values of n, l, and. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. Learn how to describe the properties of electrons in atoms using four quantum numbers: Find out the allowed values of n, l, and. Find out how to combine quantum numbers and form shells and subshells of. Principal, angular, magnetic and spin. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. The electron spin quantum number, denoted by m s. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. Principal quantum number, denoted by n. Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. Find out how they determine the energy, shape, orientation, and spin of orbitals. Find out the allowed values, symbols and examples of each. Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Magnetic quantum number, denoted by m l.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is Quantum Numbers And Orbitals There is 1 s orbital and 3 p orbitals, so there are. Find out the allowed values, symbols and examples of each. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. Find out how they determine the energy, shape, orientation, and spin of orbitals. Learn how to describe the size, shape, and orientation of atomic orbitals using. What Is Quantum Numbers And Orbitals.
From slidetodoc.com
Quantum Numbers and Orbital Diagrams Quantum Numbers Each What Is Quantum Numbers And Orbitals There is 1 s orbital and 3 p orbitals, so there are. Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Find out the allowed values, symbols and examples of each. Magnetic quantum number, denoted by m l. Learn how to describe the location and energy of electrons in atoms using quantum theory. What Is Quantum Numbers And Orbitals.
From www.albert.io
Quantum Numbers and Theory AP® Chemistry Crash Course Albert.io What Is Quantum Numbers And Orbitals Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. Magnetic quantum number, denoted by m l. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. There is. What Is Quantum Numbers And Orbitals.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is Quantum Numbers And Orbitals The electron spin quantum number, denoted by m s. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. Find out the allowed values of n, l, and. Find out the allowed values, symbols and examples of each. Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Four quantum numbers can. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Orbitals and Quantum Numbers PowerPoint Presentation, free What Is Quantum Numbers And Orbitals Find out how they determine the energy, shape, orientation, and spin of orbitals. Principal, angular, magnetic and spin. Find out the allowed values of n, l, and. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: Learn how to describe the location and energy of electrons in. What Is Quantum Numbers And Orbitals.
From www.w3schools.blog
Quantum Numbers W3schools What Is Quantum Numbers And Orbitals Find out the allowed values of n, l, and. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. There is 1 s orbital and 3 p orbitals, so there are. Explore. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID6909895 What Is Quantum Numbers And Orbitals Find out how they determine the energy, shape, orientation, and spin of orbitals. Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Find out the allowed values of n, l, and. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. Find. What Is Quantum Numbers And Orbitals.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is Quantum Numbers And Orbitals Magnetic quantum number, denoted by m l. Principal quantum number, denoted by n. There is 1 s orbital and 3 p orbitals, so there are. Principal, angular, magnetic and spin. The electron spin quantum number, denoted by m s. Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. Find out. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Is Quantum Numbers And Orbitals It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. Find out the allowed values, symbols and examples of each. Explore the concepts of principal,. Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. Find out how to combine quantum numbers. What Is Quantum Numbers And Orbitals.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Is Quantum Numbers And Orbitals Find out the allowed values, symbols and examples of each. Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. Magnetic quantum number, denoted by m l. Find out how to combine quantum numbers and form shells and subshells of. Principal, angular, magnetic and spin. Find out the allowed values of n, l,. What Is Quantum Numbers And Orbitals.
From sansona.github.io
Quantum Numbers What Is Quantum Numbers And Orbitals Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. The electron spin quantum number, denoted by m s. Find out how to combine quantum numbers and form shells and subshells of. Find out the allowed values, symbols and examples of each. Magnetic quantum number, denoted by m l. Learn how. What Is Quantum Numbers And Orbitals.
From www.vedantu.com
Quantum Numbers Azimuthal Quantum Number Types of Quantum Numbers What Is Quantum Numbers And Orbitals Principal, angular, magnetic and spin. Explore the concepts of principal,. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. Find out how they determine the energy, shape, orientation, and spin of orbitals. Find out how to combine quantum numbers and form shells and subshells of. Four quantum numbers can be. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Electron Configuration and the Periodic Table PowerPoint What Is Quantum Numbers And Orbitals Find out the allowed values of n, l, and. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. Explore the concepts of principal,. Principal quantum number, denoted by n. Principal, angular, magnetic and spin. Find out the allowed values, symbols and examples of each. Learn how to use quantum numbers. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Summary PowerPoint Presentation, free download ID6298913 What Is Quantum Numbers And Orbitals Find out the allowed values, symbols and examples of each. Principal quantum number, denoted by n. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. Principal, angular, magnetic and spin. Find out how to combine quantum numbers and form shells and subshells of. Find out the allowed values of n, l, and. There is 1 s orbital. What Is Quantum Numbers And Orbitals.
From www.geeksforgeeks.org
Quantum Numbers Principal, Azimuthal, & Spin What Is Quantum Numbers And Orbitals Find out the allowed values of n, l, and. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. There is 1 s orbital and 3 p orbitals, so there are. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom,. What Is Quantum Numbers And Orbitals.
From chem.libretexts.org
11.2 Quantum Numbers for Electrons Chemistry LibreTexts What Is Quantum Numbers And Orbitals Find out how they determine the energy, shape, orientation, and spin of orbitals. Explore the concepts of principal,. Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. Principal quantum number, denoted by n. Find out how to combine quantum numbers and form shells and subshells of. Magnetic quantum number, denoted by m. What Is Quantum Numbers And Orbitals.
From www.pearson.com
Identify the orbitals and provide the n and l quantum numbers for What Is Quantum Numbers And Orbitals The electron spin quantum number, denoted by m s. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. Magnetic quantum number, denoted by m l. Find out how to. What Is Quantum Numbers And Orbitals.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is Quantum Numbers And Orbitals Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: Find out how to combine quantum numbers and form shells and subshells of. Principal quantum number, denoted by n. The electron. What Is Quantum Numbers And Orbitals.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice What Is Quantum Numbers And Orbitals Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Find out the allowed values, symbols and examples of each. Magnetic quantum number, denoted by m l. Find out how they determine the energy, shape, orientation, and spin of orbitals. Find out the allowed values of n, l, and. Principal, angular, magnetic and spin.. What Is Quantum Numbers And Orbitals.
From www.meta-synthesis.com
Quantum Number Periodic Table Chemogenesis What Is Quantum Numbers And Orbitals The electron spin quantum number, denoted by m s. Find out the allowed values of n, l, and. Learn how to describe the properties of electrons in atoms using four quantum numbers: There is 1 s orbital and 3 p orbitals, so there are. Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of. What Is Quantum Numbers And Orbitals.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry What Is Quantum Numbers And Orbitals Magnetic quantum number, denoted by m l. Principal, angular, magnetic and spin. Find out the allowed values, symbols and examples of each. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. There is 1 s orbital and 3 p orbitals, so there are. Find out how they determine the energy,. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Lesson 18 Quantum Numbers and Electron Configurations PowerPoint What Is Quantum Numbers And Orbitals Find out how to combine quantum numbers and form shells and subshells of. Find out the allowed values, symbols and examples of each. Explore the concepts of principal,. The electron spin quantum number, denoted by m s. Find out how they determine the energy, shape, orientation, and spin of orbitals. Principal quantum number, denoted by n. There is 1 s. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 What Is Quantum Numbers And Orbitals Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. Find out how to combine quantum numbers and form shells and subshells of.. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation What Is Quantum Numbers And Orbitals Find out the allowed values, symbols and examples of each. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. The electron spin quantum number, denoted by m s. Principal quantum number, denoted by n. Learn how to describe the location and energy of electrons in atoms using quantum. What Is Quantum Numbers And Orbitals.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps What Is Quantum Numbers And Orbitals Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals.. What Is Quantum Numbers And Orbitals.
From www.researchgate.net
Principal quantum number, types and number of orbitals, maximum number What Is Quantum Numbers And Orbitals Magnetic quantum number, denoted by m l. Find out how they determine the energy, shape, orientation, and spin of orbitals. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. Principal quantum number, denoted by n. Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. There is 1 s orbital. What Is Quantum Numbers And Orbitals.
From physicscatalyst.com
Quantum Numbers Chart physicscatalyst's Blog What Is Quantum Numbers And Orbitals Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. Find out how to combine quantum numbers and form shells and subshells of. Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. Learn how to describe the properties of. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Ch. 7 Atomic and Electronic Structure PowerPoint Presentation What Is Quantum Numbers And Orbitals The electron spin quantum number, denoted by m s. Principal, angular, magnetic and spin. Find out the allowed values, symbols and examples of each. Find out how to combine quantum numbers and form shells and subshells of. Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. There is 1 s. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation What Is Quantum Numbers And Orbitals Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. There is 1 s orbital and 3 p orbitals, so there are. Find out how to combine quantum numbers and form shells. What Is Quantum Numbers And Orbitals.
From www.learninsta.com
Quantum Numbers What Is Quantum Numbers And Orbitals Principal, angular, magnetic and spin. Magnetic quantum number, denoted by m l. Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: It can have 4 orbitals, as the quantum number 2 is only possible for the. What Is Quantum Numbers And Orbitals.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is Quantum Numbers And Orbitals Learn how to describe the size, shape, and orientation of atomic orbitals using three quantum numbers: Explore the concepts of principal,. Find out the allowed values of n, l, and. Principal, angular, magnetic and spin. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. Learn how to describe. What Is Quantum Numbers And Orbitals.
From www.youtube.com
Quantum numbers and orbitals YouTube What Is Quantum Numbers And Orbitals The electron spin quantum number, denoted by m s. Find out how to combine quantum numbers and form shells and subshells of. Explore the concepts of principal,. Orbital angular momentum quantum number (or azimuthal quantum number), denoted by l. There is 1 s orbital and 3 p orbitals, so there are. Principal quantum number, denoted by n. Learn how to. What Is Quantum Numbers And Orbitals.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a What Is Quantum Numbers And Orbitals Find out how they determine the energy, shape, orientation, and spin of orbitals. Learn how to use four quantum numbers (n, l, ml, ms) to describe the movement and trajectories of electrons in atoms. Learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. Learn how to describe the properties of. What Is Quantum Numbers And Orbitals.
From www.youtube.com
3 1 3 Quantum numbers, orbitals, subshells and shells CV YouTube What Is Quantum Numbers And Orbitals Find out the allowed values of n, l, and. Find out how to combine quantum numbers and form shells and subshells of. It can have 4 orbitals, as the quantum number 2 is only possible for the s and p orbitals. The electron spin quantum number, denoted by m s. Learn how to use four quantum numbers (n, l, ml,. What Is Quantum Numbers And Orbitals.
From study.com
Quantum Numbers in Chemistry Definition, Symbol & Examples Lesson What Is Quantum Numbers And Orbitals Learn how to describe the location and energy of electrons in atoms using quantum theory and orbitals. Four quantum numbers can be used to completely describe all the attributes of a given electron belonging to an atom, these are: The electron spin quantum number, denoted by m s. Magnetic quantum number, denoted by m l. There is 1 s orbital. What Is Quantum Numbers And Orbitals.