Why Do Some Substances Not Dissolve In Water . Because of the oh group in methanol, we expect its molecules to. Not all substances are soluble in water. Why do some substances dissolve in. Solutes dissolved in water (solvent) are called aqueous solutions. Because water is polar, substances that are polar or ionic will dissolve in it. How would you design an experiment to determine the solubility of a. When a substance dissolves, it might look like it has disappeared,. What must happen in order for something to dissolve in water? Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Some substances dissolve when you mix them with water. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The hydrophobic effect is the observed tendency of nonpolar. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure.
from www.slideshare.net
Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Because of the oh group in methanol, we expect its molecules to. Not all substances are soluble in water. How would you design an experiment to determine the solubility of a. The hydrophobic effect is the observed tendency of nonpolar. What must happen in order for something to dissolve in water? The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Some substances dissolve when you mix them with water. Solutes dissolved in water (solvent) are called aqueous solutions.
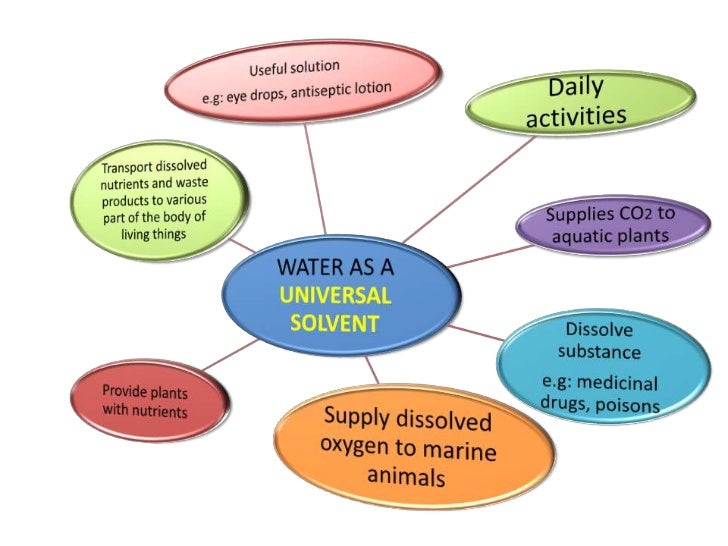
Water & solution SCIENCE FORM 2
Why Do Some Substances Not Dissolve In Water Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Because water is polar, substances that are polar or ionic will dissolve in it. Solutes dissolved in water (solvent) are called aqueous solutions. Some substances dissolve when you mix them with water. What must happen in order for something to dissolve in water? Why do some substances dissolve in. When a substance dissolves, it might look like it has disappeared,. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: How would you design an experiment to determine the solubility of a. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Not all substances are soluble in water. Because of the oh group in methanol, we expect its molecules to. The hydrophobic effect is the observed tendency of nonpolar.
From www.slideserve.com
PPT Solutions PowerPoint Presentation ID6888319 Why Do Some Substances Not Dissolve In Water Because of the oh group in methanol, we expect its molecules to. Some substances dissolve when you mix them with water. Because water is polar, substances that are polar or ionic will dissolve in it. What must happen in order for something to dissolve in water? When a substance dissolves, it might look like it has disappeared,. The hydrophobic effect. Why Do Some Substances Not Dissolve In Water.
From sciencing.com
Substances That Won't Dissolve in Water Sciencing Why Do Some Substances Not Dissolve In Water The hydrophobic effect is the observed tendency of nonpolar. Because water is polar, substances that are polar or ionic will dissolve in it. Not all substances are soluble in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Some substances dissolve when you mix them with water. When a substance dissolves, it might. Why Do Some Substances Not Dissolve In Water.
From dokumen.tips
(PPT) Intermolecular Forces and Liquids and Solids 1 Why do some solids Why Do Some Substances Not Dissolve In Water Because water is polar, substances that are polar or ionic will dissolve in it. Because of the oh group in methanol, we expect its molecules to. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: When a substance dissolves, it might look like it has disappeared,. We will first examine the process that occurs when. Why Do Some Substances Not Dissolve In Water.
From www.bbc.com
Which materials dissolve in water? BBC Bitesize Why Do Some Substances Not Dissolve In Water Solutes dissolved in water (solvent) are called aqueous solutions. What must happen in order for something to dissolve in water? The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: When a substance dissolves, it might look like it has disappeared,. Because water is polar, substances that are polar or ionic will dissolve in it. Not. Why Do Some Substances Not Dissolve In Water.
From socratic.org
Substance A will not dissolve in water. What can be said about Why Do Some Substances Not Dissolve In Water How would you design an experiment to determine the solubility of a. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: Not all substances are soluble in water. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. Because water is polar, substances that are polar or ionic. Why Do Some Substances Not Dissolve In Water.
From slidetodoc.com
HOW AND WHY DO SUBSTANCES DISSOLVE IN WATER Why Do Some Substances Not Dissolve In Water Not all substances are soluble in water. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. The hydrophobic effect is the observed tendency of nonpolar. Solutes dissolved in water (solvent) are called aqueous solutions. Because water is polar, substances that are polar or ionic will dissolve in it. Nonpolar molecules, such as. Why Do Some Substances Not Dissolve In Water.
From www.numerade.com
SOLVEDSubstances that dissolve in water generally do not dissolve in Why Do Some Substances Not Dissolve In Water How would you design an experiment to determine the solubility of a. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: Because of the oh group in methanol, we expect its molecules to. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. Some substances dissolve when you. Why Do Some Substances Not Dissolve In Water.
From www.numerade.com
SOLVED Substances that dissolve in water generally do not dissolve in Why Do Some Substances Not Dissolve In Water Because of the oh group in methanol, we expect its molecules to. Solutes dissolved in water (solvent) are called aqueous solutions. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. What must happen in order for something to dissolve in water? Not all substances are soluble in water.. Why Do Some Substances Not Dissolve In Water.
From www.youtube.com
Why oil does not dissolve in water Polarity of water YouTube Why Do Some Substances Not Dissolve In Water Because of the oh group in methanol, we expect its molecules to. How would you design an experiment to determine the solubility of a. Because water is polar, substances that are polar or ionic will dissolve in it. The hydrophobic effect is the observed tendency of nonpolar. Some substances dissolve when you mix them with water. The hydrophobic effect can. Why Do Some Substances Not Dissolve In Water.
From dokumen.tips
(PPT) Why do some solids dissolve in water but others do not? Why are Why Do Some Substances Not Dissolve In Water Not all substances are soluble in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When a substance dissolves, it might look like it has disappeared,. The ability of substances to dissolve. Why Do Some Substances Not Dissolve In Water.
From slideplayer.com
Properties of Water Chapter ppt download Why Do Some Substances Not Dissolve In Water Why do some substances dissolve in. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. The hydrophobic effect is the observed tendency of nonpolar. When a substance dissolves, it might look like it has disappeared,. How would you design an experiment to determine the solubility of a. The hydrophobic effect can better. Why Do Some Substances Not Dissolve In Water.
From www.slideshare.net
Water & solution SCIENCE FORM 2 Why Do Some Substances Not Dissolve In Water Why do some substances dissolve in. When a substance dissolves, it might look like it has disappeared,. Solutes dissolved in water (solvent) are called aqueous solutions. Some substances dissolve when you mix them with water. The hydrophobic effect is the observed tendency of nonpolar. Because water is polar, substances that are polar or ionic will dissolve in it. We will. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Chemical and Physical Features of Seawater and the World Ocean Why Do Some Substances Not Dissolve In Water What must happen in order for something to dissolve in water? The hydrophobic effect is the observed tendency of nonpolar. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. Solutes dissolved. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Chapter 5 Solutions PowerPoint Presentation, free download ID Why Do Some Substances Not Dissolve In Water Some substances dissolve when you mix them with water. How would you design an experiment to determine the solubility of a. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Solutes dissolved in water (solvent) are called aqueous solutions. The ability of substances to dissolve in water depends. Why Do Some Substances Not Dissolve In Water.
From www.learnersplanet.com
6th cbse science separation of substances class notes Why Do Some Substances Not Dissolve In Water Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. How would you design an experiment to determine the solubility of a. The hydrophobic effect is the observed tendency of nonpolar. What must happen in order for something to dissolve in water? We will first examine the process that occurs when an ionic compound, such. Why Do Some Substances Not Dissolve In Water.
From sciencing.com
Substances That Won't Dissolve in Water Sciencing Why Do Some Substances Not Dissolve In Water Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. The hydrophobic effect is the observed tendency of nonpolar. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: When a substance dissolves, it might look like it has disappeared,. What must happen in order for something to dissolve in water?. Why Do Some Substances Not Dissolve In Water.
From exorlozgr.blob.core.windows.net
What Substances Do Not Dissolve In Water at Margaret Leary blog Why Do Some Substances Not Dissolve In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Because water is polar, substances that are polar or ionic will dissolve in it. Not all substances are soluble in water. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: When a substance dissolves,. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free Why Do Some Substances Not Dissolve In Water How would you design an experiment to determine the solubility of a. What must happen in order for something to dissolve in water? Because water is polar, substances that are polar or ionic will dissolve in it. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: Solutes dissolved in water (solvent) are called aqueous solutions.. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT SOLUTIONS PowerPoint Presentation, free download ID3208141 Why Do Some Substances Not Dissolve In Water The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. Solutes dissolved in water (solvent) are called aqueous solutions. Why do some substances dissolve in. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: What must happen in order for something to dissolve in water? Nonpolar molecules, such. Why Do Some Substances Not Dissolve In Water.
From lessonlibunidealism.z13.web.core.windows.net
Water As A Universal Solvent Notes Why Do Some Substances Not Dissolve In Water The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. Because water is polar, substances that are polar or ionic will dissolve in it. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. How would you design an experiment to determine. Why Do Some Substances Not Dissolve In Water.
From slideplayer.com
What is it and how does it work? ppt download Why Do Some Substances Not Dissolve In Water Because of the oh group in methanol, we expect its molecules to. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Not all substances are soluble in water. Because water is polar, substances that are polar or ionic will dissolve in it. What must happen in order for. Why Do Some Substances Not Dissolve In Water.
From brainly.in
the substance that will not dissolve in water Brainly.in Why Do Some Substances Not Dissolve In Water Why do some substances dissolve in. The hydrophobic effect is the observed tendency of nonpolar. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: Not all substances are soluble in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. What must happen. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Water, Water, Everywhere (Ch. 3) PowerPoint Presentation, free Why Do Some Substances Not Dissolve In Water Solutes dissolved in water (solvent) are called aqueous solutions. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Some substances dissolve when you mix them with water. Why do some substances dissolve in. Not all substances are soluble in water. What must happen in order for something to dissolve in water? The ability of. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 Why Do Some Substances Not Dissolve In Water When a substance dissolves, it might look like it has disappeared,. Some substances dissolve when you mix them with water. Why do some substances dissolve in. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: What must happen in order for something to dissolve in water? The hydrophobic effect is the observed tendency of nonpolar.. Why Do Some Substances Not Dissolve In Water.
From slideplayer.com
Solutions Chapter ppt download Why Do Some Substances Not Dissolve In Water How would you design an experiment to determine the solubility of a. Not all substances are soluble in water. Some substances dissolve when you mix them with water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure.. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Types of Chemical Reactions & Solutions PowerPoint Presentation Why Do Some Substances Not Dissolve In Water Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Not all substances are soluble in water. Solutes dissolved in water (solvent) are called aqueous solutions. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. When a substance dissolves, it might look like it has disappeared,. What. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Why Do Some Substances Not Dissolve In Water Because water is polar, substances that are polar or ionic will dissolve in it. How would you design an experiment to determine the solubility of a. Because of the oh group in methanol, we expect its molecules to. Solutes dissolved in water (solvent) are called aqueous solutions. Not all substances are soluble in water. The hydrophobic effect is the observed. Why Do Some Substances Not Dissolve In Water.
From www.timesnownews.com
Why Do Some Substances Dissolve In Water? Explained Explainers News Why Do Some Substances Not Dissolve In Water Solutes dissolved in water (solvent) are called aqueous solutions. The hydrophobic effect is the observed tendency of nonpolar. Because of the oh group in methanol, we expect its molecules to. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: What must happen in order for something to dissolve in water? Why do some substances dissolve. Why Do Some Substances Not Dissolve In Water.
From publicaffairsworld.com
what dissolves in water science project Why Do Some Substances Not Dissolve In Water Because water is polar, substances that are polar or ionic will dissolve in it. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Because of the oh group in methanol, we expect its molecules to. When a substance dissolves, it might look like it has disappeared,. The ability. Why Do Some Substances Not Dissolve In Water.
From www.youtube.com
Why do some substances dissolve in water and others do not? YouTube Why Do Some Substances Not Dissolve In Water Because of the oh group in methanol, we expect its molecules to. Not all substances are soluble in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: How would you design an experiment. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Solutes, Solutions and Solvents PowerPoint Presentation ID2528981 Why Do Some Substances Not Dissolve In Water Not all substances are soluble in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Solutes dissolved in water (solvent) are called aqueous solutions. Because of the oh group in methanol, we expect its molecules to. Some substances dissolve when you mix them with water. The hydrophobic. Why Do Some Substances Not Dissolve In Water.
From byjus.com
Which among the following things do not dissolve in water? Why Do Some Substances Not Dissolve In Water What must happen in order for something to dissolve in water? Why do some substances dissolve in. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When a substance dissolves, it might look like it has disappeared,. Solutes dissolved in water (solvent) are called aqueous solutions. Some substances. Why Do Some Substances Not Dissolve In Water.
From shaunmwilliams.com
Chapter 3 Presentation Why Do Some Substances Not Dissolve In Water The hydrophobic effect can better explain why some nonpolar molecules can't dissolve in water: When a substance dissolves, it might look like it has disappeared,. Because water is polar, substances that are polar or ionic will dissolve in it. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. How would you design an experiment. Why Do Some Substances Not Dissolve In Water.
From www.slideserve.com
PPT Unit 6 TOXINS Solutions & PowerPoint Presentation ID Why Do Some Substances Not Dissolve In Water Why do some substances dissolve in. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When a substance dissolves, it might look like it has disappeared,. The ability of substances to dissolve in water depends on factors like their polarity, size, and structure. Not all substances are soluble in water. The hydrophobic effect can. Why Do Some Substances Not Dissolve In Water.
From www.slideshare.net
Solubility (a physical property) (Teach) Why Do Some Substances Not Dissolve In Water Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Solutes dissolved in water (solvent) are called aqueous solutions. The hydrophobic effect is the observed tendency of nonpolar. Not all substances are soluble in water. Because water is polar, substances that are polar or ionic will dissolve in it. Some substances dissolve when you mix. Why Do Some Substances Not Dissolve In Water.