How Do You Calculate Standard Enthalpy Of Formation . Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The standard state of an element can be identified in. State the first law of thermodynamics. By the end of this section, you will be able to:
from general.chemistrysteps.com
The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. By the end of this section, you will be able to: 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. State the first law of thermodynamics. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard state of an element can be identified in.
Standard Enthalpies of Formation Chemistry Steps
How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state of an element can be identified in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. By the end of this section, you will be able to: The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. State the first law of thermodynamics.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard How Do You Calculate Standard Enthalpy Of Formation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. State the first law of thermodynamics. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. Standard enthalpy of formation (or heat of. How Do You Calculate Standard Enthalpy Of Formation.
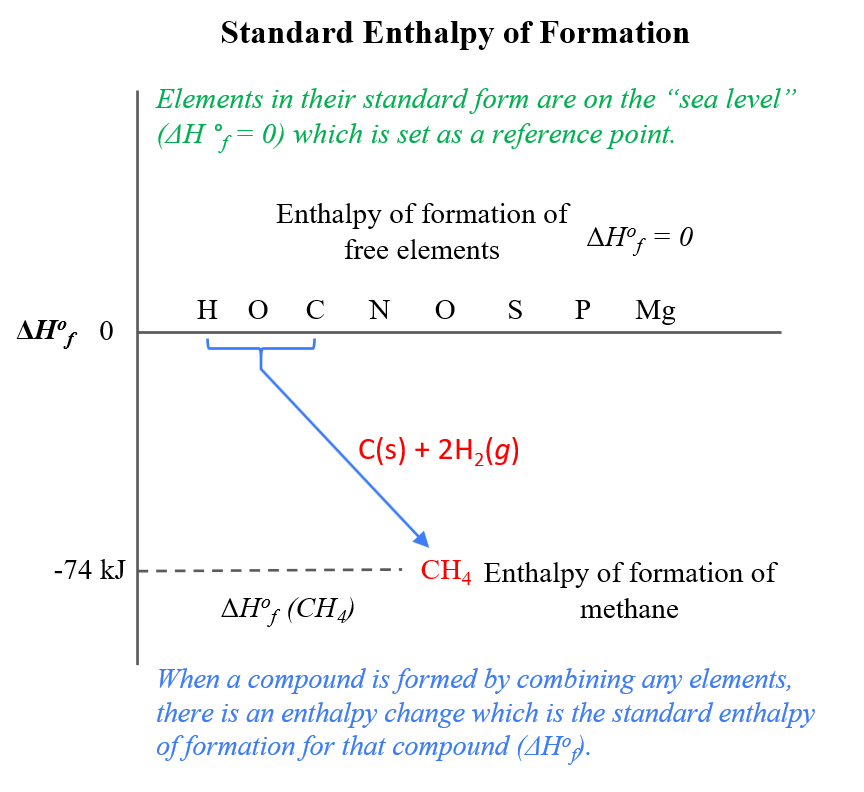
From studylib.net
Standard Enthalpy of Formation and Reaction How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. By the end of this section, you will be able to: State the first law of thermodynamics. To calculate the standard enthalpy of formation of. How Do You Calculate Standard Enthalpy Of Formation.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib How Do You Calculate Standard Enthalpy Of Formation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation (or heat of formation), δhof ,. How Do You Calculate Standard Enthalpy Of Formation.
From mungfali.com
Standard Enthalpy Change Equation How Do You Calculate Standard Enthalpy Of Formation To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state of an element can be identified in. 193 rows the standard. How Do You Calculate Standard Enthalpy Of Formation.
From mungfali.com
Enthalpy Change Of Formation Equation How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. State the first law. How Do You Calculate Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in How Do You Calculate Standard Enthalpy Of Formation By the end of this section, you will be able to: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard molar. How Do You Calculate Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard. How Do You Calculate Standard Enthalpy Of Formation.
From diagramlibraryisadore.z21.web.core.windows.net
How To Calculate The Enthalpy Change How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. How Do You Calculate Standard Enthalpy Of Formation.
From www.youtube.com
Calculating Reaction Enthalpy from Enthalpies of Formation YouTube How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants. How Do You Calculate Standard Enthalpy Of Formation.
From www.youtube.com
How to Calculate Enthalpy of Reaction using Heat of Formation Examples How Do You Calculate Standard Enthalpy Of Formation 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. State the first law of thermodynamics. The standard state of an element can be identified in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from. How Do You Calculate Standard Enthalpy Of Formation.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation How Do You Calculate Standard Enthalpy Of Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard state of an element can be identified in. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. 193 rows the standard enthalpy change. How Do You Calculate Standard Enthalpy Of Formation.
From www.slideshare.net
Standard enthalpy of formation How Do You Calculate Standard Enthalpy Of Formation To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. State the first law of thermodynamics. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows the standard enthalpy change of any reaction can. How Do You Calculate Standard Enthalpy Of Formation.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$. How Do You Calculate Standard Enthalpy Of Formation.
From www.thestudentroom.co.uk
Calculate standard enthalpy of formation from thermodynamic data? The How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants. How Do You Calculate Standard Enthalpy Of Formation.
From byjus.com
21. At 300 K standard enthalpy of formation of C6H5COOH(s), CO2(g), and How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. How Do You Calculate Standard Enthalpy Of Formation.
From www.numerade.com
(a) Calculate the standard enthalpy of formation of gaseous diborane How Do You Calculate Standard Enthalpy Of Formation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard state of an element can be identified in. State the first law. How Do You Calculate Standard Enthalpy Of Formation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps How Do You Calculate Standard Enthalpy Of Formation 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. State the first law. How Do You Calculate Standard Enthalpy Of Formation.
From lessonmagictirolese.z14.web.core.windows.net
How To Do Enthalpy Equations How Do You Calculate Standard Enthalpy Of Formation To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change. How Do You Calculate Standard Enthalpy Of Formation.
From www.toppr.com
Calculate the standard enthalpy of formation of CH3 OH from the How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent. How Do You Calculate Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. How Do You Calculate Standard Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. State the first law. How Do You Calculate Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. State the first law of thermodynamics. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance. How Do You Calculate Standard Enthalpy Of Formation.
From mungfali.com
Standard Enthalpy Of Formation Equation How Do You Calculate Standard Enthalpy Of Formation State the first law of thermodynamics. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard. How Do You Calculate Standard Enthalpy Of Formation.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy How Do You Calculate Standard Enthalpy Of Formation To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. State the first law of thermodynamics. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard state of. How Do You Calculate Standard Enthalpy Of Formation.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table How Do You Calculate Standard Enthalpy Of Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state of an element can be identified in.. How Do You Calculate Standard Enthalpy Of Formation.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation How Do You Calculate Standard Enthalpy Of Formation By the end of this section, you will be able to: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. How Do You Calculate Standard Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A. How Do You Calculate Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of How Do You Calculate Standard Enthalpy Of Formation The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A. How Do You Calculate Standard Enthalpy Of Formation.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction How Do You Calculate Standard Enthalpy Of Formation State the first law of thermodynamics. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. By the end. How Do You Calculate Standard Enthalpy Of Formation.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of How Do You Calculate Standard Enthalpy Of Formation Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. State the first law of thermodynamics. By the end of. How Do You Calculate Standard Enthalpy Of Formation.
From studylib.net
Using Standard Molar Enthalpies of Formation to Calculate Enthalpy How Do You Calculate Standard Enthalpy Of Formation To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. By the end of this section, you will be able to: 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. Standard enthalpy of formation (or heat of formation),. How Do You Calculate Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies How Do You Calculate Standard Enthalpy Of Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. By the end of this section, you will be able to: To calculate the standard enthalpy of formation of a compound, we must start with the elements in their standard states. The standard molar enthalpy of. How Do You Calculate Standard Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube How Do You Calculate Standard Enthalpy Of Formation The standard state of an element can be identified in. State the first law of thermodynamics. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard molar enthalpy of formation δh o f is the enthalpy change when. How Do You Calculate Standard Enthalpy Of Formation.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy How Do You Calculate Standard Enthalpy Of Formation By the end of this section, you will be able to: The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed from its elements in. The standard enthalpy of formation is a measure of the energy released or consumed. How Do You Calculate Standard Enthalpy Of Formation.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine How Do You Calculate Standard Enthalpy Of Formation 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. By the end of this section, you will be able to: State the first law of thermodynamics. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed. How Do You Calculate Standard Enthalpy Of Formation.