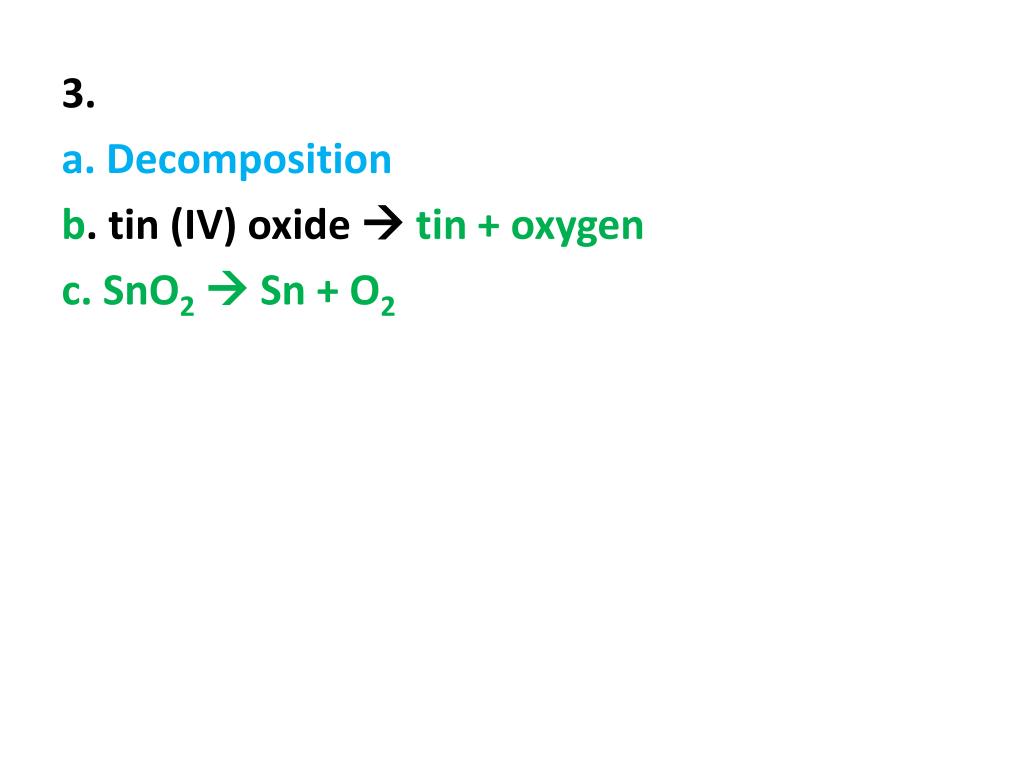Tin Iv Oxide Decomposition . The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Sublimes between 1800°c and 1900°c. A white solid, sno 2, insoluble in water; Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. 6.95 g/cm3 insoluble in water. According to diraction data presented in.
from www.slideserve.com
6.95 g/cm3 insoluble in water. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. According to diraction data presented in. Sublimes between 1800°c and 1900°c. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. A white solid, sno 2, insoluble in water;
PPT Directions For each of the following Classify the type of
Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. 6.95 g/cm3 insoluble in water. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. Sublimes between 1800°c and 1900°c. According to diraction data presented in. A white solid, sno 2, insoluble in water; The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in.
From www.semanticscholar.org
Figure 12 from Facile Fabrication of Au Nanoparticles/Tin Oxide/Reduced Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. According to diraction data presented in. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders were obtained by decomposition of tin (ii). Tin Iv Oxide Decomposition.
From www.researchgate.net
TGA analysis of Tin (IV) oxide (a) and Sulfated tin (IV) oxide (b Tin Iv Oxide Decomposition According to diraction data presented in. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. A white solid, sno 2, insoluble in water; The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. Sublimes between 1800°c. Tin Iv Oxide Decomposition.
From www.chegg.com
Solved Consider the reaction of tin(IV) oxide with carbon. Tin Iv Oxide Decomposition A white solid, sno 2, insoluble in water; Sublimes between 1800°c and 1900°c. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. The synthesis of tin (iv) oxide by. Tin Iv Oxide Decomposition.
From www.researchgate.net
(PDF) Evaluation the performance of the tin (IV) oxide (SnO2) in the Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. A white solid, sno 2, insoluble in water; The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders were obtained by decomposition of. Tin Iv Oxide Decomposition.
From www.numerade.com
Smelting When tin(IV) oxide is heated with carbon in a process called Tin Iv Oxide Decomposition According to diraction data presented in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. 6.95 g/cm3 insoluble in water. Sublimes between 1800°c and 1900°c. The decomposition of the 4d. Tin Iv Oxide Decomposition.
From www.youtube.com
How to write chemical formula Tin IV Oxide YouTube Tin Iv Oxide Decomposition Sublimes between 1800°c and 1900°c. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. 6.95 g/cm3 insoluble in water. According to diraction data presented in. The decomposition of the 4d. Tin Iv Oxide Decomposition.
From journals.sagepub.com
Dyes catalytic degradation using modified tin(IV) oxide and hydroxide Tin Iv Oxide Decomposition 6.95 g/cm3 insoluble in water. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. According to diraction data presented in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. A white solid, sno 2, insoluble in water; The decomposition. Tin Iv Oxide Decomposition.
From www.studypool.com
SOLUTION Isothermal thermo analytical study and Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. Sublimes between 1800°c and 1900°c. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition. Tin Iv Oxide Decomposition.
From www.studypool.com
SOLUTION Isothermal thermo analytical study and Tin Iv Oxide Decomposition A white solid, sno 2, insoluble in water; Sublimes between 1800°c and 1900°c. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. According to diraction data presented in. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. The decomposition. Tin Iv Oxide Decomposition.
From inspiritvr.com
Reaction Study Guide Inspirit Tin Iv Oxide Decomposition The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. According to diraction data presented in. A white solid, sno 2, insoluble in water; Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The decomposition of the 4d 3/2 and. Tin Iv Oxide Decomposition.
From www.fishersci.ie
Potassium tin(IV) oxide trihydrate, 95, Thermo Scientific Chemicals Tin Iv Oxide Decomposition Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. A white solid, sno 2, insoluble in water; 6.95 g/cm3 insoluble in water. According to diraction data presented in. Sublimes between. Tin Iv Oxide Decomposition.
From www.slideserve.com
PPT Directions For each of the following Classify the type of Tin Iv Oxide Decomposition Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. According to diraction data presented in. 6.95 g/cm3 insoluble in water. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. The decomposition of the 4d 3/2 and 4d 5/2 signals. Tin Iv Oxide Decomposition.
From www.alamy.com
Full labelled diagram of the laboratory preparation of oxygen from Tin Iv Oxide Decomposition A white solid, sno 2, insoluble in water; According to diraction data presented in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Sublimes between 1800°c and 1900°c. The decomposition. Tin Iv Oxide Decomposition.
From pubs.acs.org
Thermal of Tin(II) Oxyhydroxide and Subsequent Oxidation Tin Iv Oxide Decomposition According to diraction data presented in. Sublimes between 1800°c and 1900°c. 6.95 g/cm3 insoluble in water. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. A white solid, sno 2,. Tin Iv Oxide Decomposition.
From www.cell.com
Epoxidation of Argemone mexicana oil with peroxyacetic acid formed in Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. A white solid, sno 2, insoluble in water; Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. 6.95 g/cm3 insoluble in water. According to diraction data presented in. The. Tin Iv Oxide Decomposition.
From www.semanticscholar.org
Figure 1 from Facile Fabrication of Au Nanoparticles/Tin Oxide/Reduced Tin Iv Oxide Decomposition According to diraction data presented in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. 6.95 g/cm3 insoluble in water. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Sublimes between 1800°c and 1900°c. A white solid, sno 2,. Tin Iv Oxide Decomposition.
From slideplayer.com
The Chemistry of Germanium, Tin and Lead ppt download Tin Iv Oxide Decomposition The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. According to diraction data presented in. Tin (iv) oxide powders were obtained by decomposition of tin (ii). Tin Iv Oxide Decomposition.
From www.semanticscholar.org
Figure 1 from Sensitization of Carbon Doped Tin (IV) Oxide Tin Iv Oxide Decomposition Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. According to diraction data presented in. A white solid, sno 2, insoluble in water; The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Sublimes between 1800°c and 1900°c. 6.95 g/cm3. Tin Iv Oxide Decomposition.
From www.semanticscholar.org
Figure 11 from Facile Fabrication of Au Nanoparticles/Tin Oxide/Reduced Tin Iv Oxide Decomposition 6.95 g/cm3 insoluble in water. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. According to diraction data presented in. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. A white solid, sno 2, insoluble in water; The decomposition. Tin Iv Oxide Decomposition.
From www.youtube.com
How to Write the Formula for Tin (IV) oxide YouTube Tin Iv Oxide Decomposition The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. According to diraction data presented in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. Sublimes between 1800°c and 1900°c. 6.95 g/cm3 insoluble in water. A white solid, sno 2,. Tin Iv Oxide Decomposition.
From loeqyugeg.blob.core.windows.net
Copper(Ii) Oxide And Carbon Equation at John Wyllie blog Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. 6.95 g/cm3 insoluble in water. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Sublimes between 1800°c and 1900°c. A white solid, sno 2, insoluble in. Tin Iv Oxide Decomposition.
From sciencekitstore.com
Tin Oxide, Stannic Oxide, Tin (IV) Oxide, SnO2, 99.8 Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. According to diraction data presented in. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Sublimes between 1800°c and 1900°c. A white solid, sno 2, insoluble. Tin Iv Oxide Decomposition.
From www.metallurgyfordummies.com
What is Indium Tin Oxide? Metallurgy for Dummies Tin Iv Oxide Decomposition The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. Sublimes between 1800°c and 1900°c. A white solid, sno 2, insoluble in water; According to diraction data presented in. The decomposition. Tin Iv Oxide Decomposition.
From www.cell.com
Epoxidation of Argemone mexicana oil with peroxyacetic acid formed in Tin Iv Oxide Decomposition 6.95 g/cm3 insoluble in water. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. A white solid, sno 2, insoluble in water; According to diraction data presented in. The decomposition. Tin Iv Oxide Decomposition.
From www.studypool.com
SOLUTION Isothermal thermo analytical study and Tin Iv Oxide Decomposition The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. According to diraction data presented in. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify. Tin Iv Oxide Decomposition.
From www.alamy.com
Tin iv oxide hires stock photography and images Alamy Tin Iv Oxide Decomposition According to diraction data presented in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Sublimes between 1800°c and 1900°c. A white solid, sno 2, insoluble in water; The decomposition. Tin Iv Oxide Decomposition.
From www.numerade.com
SOLVED For the equation SnO2 + 2H2 Sn + 2H2O, tin (IV) oxide reacts Tin Iv Oxide Decomposition Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. According to diraction data presented in. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. The synthesis of tin (iv) oxide by cvd method was carried out by thermal. Tin Iv Oxide Decomposition.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID2670505 Tin Iv Oxide Decomposition Sublimes between 1800°c and 1900°c. 6.95 g/cm3 insoluble in water. A white solid, sno 2, insoluble in water; The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The decomposition of. Tin Iv Oxide Decomposition.
From www.researchgate.net
SEM images of tin (IV) oxide (a) and sulfated tin (IV) oxide (b Tin Iv Oxide Decomposition 6.95 g/cm3 insoluble in water. Sublimes between 1800°c and 1900°c. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. According to diraction data presented in. A white solid, sno 2, insoluble in water; The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence. Tin Iv Oxide Decomposition.
From www.slideserve.com
PPT Synthesis and Reactions PowerPoint Presentation Tin Iv Oxide Decomposition A white solid, sno 2, insoluble in water; The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. Sublimes between 1800°c and 1900°c. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. According to diraction data. Tin Iv Oxide Decomposition.
From www.researchgate.net
Enthalpy of formation, reaction, and of metal oxides and Tin Iv Oxide Decomposition The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. According to diraction data presented in. 6.95 g/cm3 insoluble in water. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. The decomposition of the 4d 3/2 and 4d 5/2 signals. Tin Iv Oxide Decomposition.
From chemcraft.su
Tin(IV) oxide, 99.9 pure p.a. chemcraft.su Tin Iv Oxide Decomposition The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. 6.95 g/cm3 insoluble in water. A white solid, sno 2, insoluble in water; The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. Tin (iv) oxide powders. Tin Iv Oxide Decomposition.
From www.onlinemathlearning.com
of Metal Compounds (solutions, examples, activities Tin Iv Oxide Decomposition Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223 k and 1323 k. 6.95 g/cm3 insoluble in water. A white solid, sno 2, insoluble in water; According to diraction data presented in. The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. The decomposition. Tin Iv Oxide Decomposition.
From www.americanelements.com
Tin(IV) Oxide AMERICAN ELEMENTS Tin Iv Oxide Decomposition The synthesis of tin (iv) oxide by cvd method was carried out by thermal decomposition of tin (ii) oxalate precursor in. A white solid, sno 2, insoluble in water; The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. 6.95 g/cm3 insoluble in water. Sublimes between 1800°c and. Tin Iv Oxide Decomposition.
From slideplayer.com
When comparing enthalpy changes for formation reactions of different Tin Iv Oxide Decomposition Sublimes between 1800°c and 1900°c. The decomposition of the 4d 3/2 and 4d 5/2 signals can be used to identify the simultaneous presence of sn(ii) and sn(iv),. A white solid, sno 2, insoluble in water; According to diraction data presented in. 6.95 g/cm3 insoluble in water. Tin (iv) oxide powders were obtained by decomposition of tin (ii) oxalate at 1223. Tin Iv Oxide Decomposition.