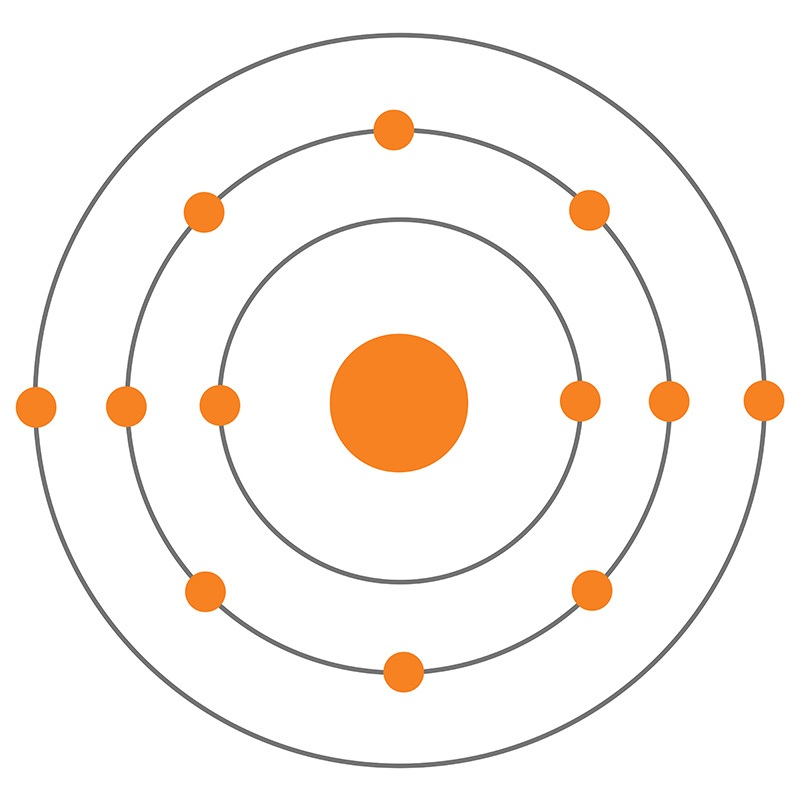
The Magnesium Bohr Model . In the nucleus you would show the 12. Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. Atomic mass, electron configurations, charges, and more. Magnesium has 3 energy levels: View rotating bohr models for all 118 elements. Define an energy level in terms of the bohr model. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. Describe the arrangement of electrons using the shell model. Discuss how the bohr model can be used to explain atomic spectra. The first electron shell of a bohr model holds 2 electrons. 119 rows access detailed info on all elements:
from mavink.com
Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. View rotating bohr models for all 118 elements. Magnesium has 3 energy levels: Discuss how the bohr model can be used to explain atomic spectra. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. In the nucleus you would show the 12. The first electron shell of a bohr model holds 2 electrons. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. 119 rows access detailed info on all elements:
Magnesium Bohr Model Diagram
The Magnesium Bohr Model Atomic mass, electron configurations, charges, and more. Discuss how the bohr model can be used to explain atomic spectra. Magnesium has 3 energy levels: The first electron shell of a bohr model holds 2 electrons. Define an energy level in terms of the bohr model. View rotating bohr models for all 118 elements. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Describe the arrangement of electrons using the shell model. In the nucleus you would show the 12. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. 119 rows access detailed info on all elements: So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. Atomic mass, electron configurations, charges, and more.
From mungfali.com
Magnesium Bohr Model The Magnesium Bohr Model View rotating bohr models for all 118 elements. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. 119 rows access detailed info on all elements: The first electron shell of a bohr model holds 2 electrons. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. Define. The Magnesium Bohr Model.
From ar.inspiredpencil.com
Magnesium Atom Structure The Magnesium Bohr Model Magnesium has 3 energy levels: Describe the arrangement of electrons using the shell model. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The first electron shell of a bohr model holds 2 electrons. Atomic mass, electron. The Magnesium Bohr Model.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image The Magnesium Bohr Model In the nucleus you would show the 12. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. Discuss how the bohr model can be used to explain atomic spectra. View rotating bohr models for all 118 elements. Describe the arrangement of electrons. The Magnesium Bohr Model.
From www.shutterstock.com
Bohr Model Magnesium Atom Electron Structure Stock Vector (Royalty Free The Magnesium Bohr Model In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. Discuss how the bohr model can be used to explain atomic spectra. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Describe the arrangement of. The Magnesium Bohr Model.
From www.slideserve.com
PPT Bohr Models PowerPoint Presentation, free download ID949135 The Magnesium Bohr Model 119 rows access detailed info on all elements: Define an energy level in terms of the bohr model. The first electron shell of a bohr model holds 2 electrons. Discuss how the bohr model can be used to explain atomic spectra. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which. The Magnesium Bohr Model.
From schematicfixlankier.z21.web.core.windows.net
Bohr Diagram For Magnesium The Magnesium Bohr Model Magnesium has 3 energy levels: The first electron shell of a bohr model holds 2 electrons. Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. In the nucleus you would show the 12. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. Define an energy level in. The Magnesium Bohr Model.
From mavink.com
Magnesium Bohr Model Diagram The Magnesium Bohr Model In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Describe the arrangement of electrons using the shell model. Define an energy level in terms of the bohr model. Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. The first electron shell of a bohr. The Magnesium Bohr Model.
From www.animalia-life.club
Bohr Model Labeled The Magnesium Bohr Model Define an energy level in terms of the bohr model. Magnesium has 3 energy levels: Discuss how the bohr model can be used to explain atomic spectra. Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. In this video we'll look at the atomic structure and bohr model for the magnesium atom. The Magnesium Bohr Model.
From mavink.com
Magnesium Bohr Model Diagram The Magnesium Bohr Model In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Describe the arrangement of electrons using the shell model. Atomic mass, electron configurations, charges, and more. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. So far, 10 of magnesium's 12 electrons have been. The Magnesium Bohr Model.
From www.americanelements.com
Magnesium (Mg) AMERICAN ELEMENTSs The Magnesium Bohr Model Atomic mass, electron configurations, charges, and more. Define an energy level in terms of the bohr model. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). View rotating bohr models for all 118 elements. In the nucleus you would show the 12. So far, 10 of magnesium's 12 electrons have been used,. The Magnesium Bohr Model.
From ffionhooper.blogspot.com
11+ Bohr Diagram Mg FfionHooper The Magnesium Bohr Model 119 rows access detailed info on all elements: 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. Define an energy level in terms of the bohr model. The first electron shell of a bohr model holds 2 electrons. View rotating bohr models for all 118 elements. Discuss how the bohr model. The Magnesium Bohr Model.
From brainly.com
This is Bohr's Model of A. Oxygen B. Magnesium C. Sulfur The Magnesium Bohr Model The first electron shell of a bohr model holds 2 electrons. 119 rows access detailed info on all elements: In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table. The Magnesium Bohr Model.
From mungfali.com
Magnesium Atom Bohr Model The Magnesium Bohr Model View rotating bohr models for all 118 elements. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. Define an energy level in terms of the bohr model. 119 rows access detailed info on all elements: In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons. The Magnesium Bohr Model.
From stock.adobe.com
Bohr model representation of the magnesium atom, number 12 and symbol The Magnesium Bohr Model In the nucleus you would show the 12. Atomic mass, electron configurations, charges, and more. Discuss how the bohr model can be used to explain atomic spectra. Describe the arrangement of electrons using the shell model. 119 rows access detailed info on all elements: So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. 2 electrons. The Magnesium Bohr Model.
From www.showme.com
Bohr Model for Magnesium Science, Chemistry, Atoms, Elements ShowMe The Magnesium Bohr Model In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. View rotating bohr models for all 118 elements. Discuss how the bohr model can be used to explain atomic spectra. In this video we'll look at the atomic structure and bohr model for. The Magnesium Bohr Model.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background The Magnesium Bohr Model In the nucleus you would show the 12. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118 elements. Explore the interactive simulation to build and understand atoms,. The Magnesium Bohr Model.
From www.shutterstock.com
Representación del modelo Bohr del átomo vector de stock (libre de The Magnesium Bohr Model 119 rows access detailed info on all elements: Magnesium has 3 energy levels: Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. Atomic mass, electron configurations, charges, and more. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. Define an energy level in terms of the bohr. The Magnesium Bohr Model.
From keywordsuggest.org
Image Gallery magnesium model The Magnesium Bohr Model So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. The first electron shell of a bohr model holds 2 electrons. In the nucleus you would show the 12. Atomic mass, electron configurations, charges, and more. 119 rows access detailed info on all elements: Explore the interactive simulation to build and understand atoms, isotopes, and their. The Magnesium Bohr Model.
From la.wikipedia.org
FasciculusElectron shell 012 magnesium.png Vicipaedia The Magnesium Bohr Model Magnesium has 3 energy levels: So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. The first electron shell of a bohr model holds 2 electrons. 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. In this video we'll look at the atomic structure and bohr model for the magnesium. The Magnesium Bohr Model.
From www.freepik.com
Premium Vector Magnesium atom bohr model The Magnesium Bohr Model The first electron shell of a bohr model holds 2 electrons. 119 rows access detailed info on all elements: Define an energy level in terms of the bohr model. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table. The Magnesium Bohr Model.
From cartoondealer.com
Magnesium Atom Bohr Model Cartoon Vector 267662111 The Magnesium Bohr Model Discuss how the bohr model can be used to explain atomic spectra. 119 rows access detailed info on all elements: The first electron shell of a bohr model holds 2 electrons. Magnesium has 3 energy levels: In the nucleus you would show the 12. In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an. The Magnesium Bohr Model.
From mungfali.com
Magnesium Bohr Model The Magnesium Bohr Model View rotating bohr models for all 118 elements. The first electron shell of a bohr model holds 2 electrons. Magnesium has 3 energy levels: Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. 119 rows access detailed info on all elements: So far, 10 of magnesium's 12 electrons have been used, so. The Magnesium Bohr Model.
From stock.adobe.com
Bohr model diagram of magnesium in atomic physics Stock Vector Adobe The Magnesium Bohr Model 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. Define an energy level in terms of the bohr model. 119 rows access detailed info on all elements: Magnesium has 3 energy levels: View rotating bohr models for. The Magnesium Bohr Model.
From cartoondealer.com
Magnesium Atom Bohr Model Cartoon Vector 267662111 The Magnesium Bohr Model Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. 119 rows access detailed info on all elements:. The Magnesium Bohr Model.
From www.dreamstime.com
Bohr Model of the Magnesium Atom Stock Illustration Illustration of The Magnesium Bohr Model In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. In the nucleus you would show the 12. Magnesium has 3 energy levels: View rotating bohr models for all 118 elements. The first electron shell of a bohr model holds 2 electrons. Discuss. The Magnesium Bohr Model.
From www.shutterstock.com
14 Magnesio Modelo De Bohr Images, Stock Photos, 3D objects, & Vectors The Magnesium Bohr Model In 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. Describe the arrangement of electrons using the shell model. Discuss how the bohr model can be used to explain atomic spectra. Explore the interactive simulation to build and understand atoms, isotopes, and their. The Magnesium Bohr Model.
From guidediagramdiptera.z22.web.core.windows.net
Bohr Diagram Of Mg The Magnesium Bohr Model Describe the arrangement of electrons using the shell model. Magnesium has 3 energy levels: View rotating bohr models for all 118 elements. Discuss how the bohr model can be used to explain atomic spectra. The first electron shell of a bohr model holds 2 electrons. Atomic mass, electron configurations, charges, and more. In 1913, the danish physicist niels bohr proposed. The Magnesium Bohr Model.
From www.shutterstock.com
Bohr Model Magnesium Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า The Magnesium Bohr Model So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. In this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. The first electron shell of a bohr model holds 2 electrons. Atomic. The Magnesium Bohr Model.
From ar.inspiredpencil.com
Magnesium Bohr Model The Magnesium Bohr Model Magnesium has 3 energy levels: View rotating bohr models for all 118 elements. Discuss how the bohr model can be used to explain atomic spectra. Define an energy level in terms of the bohr model. In the nucleus you would show the 12. Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet.. The Magnesium Bohr Model.
From www.slideserve.com
PPT Bohr models PowerPoint Presentation, free download ID3062580 The Magnesium Bohr Model Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. Magnesium has 3 energy levels: 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. So far, 10 of magnesium's. The Magnesium Bohr Model.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and The Magnesium Bohr Model Explore the interactive simulation to build and understand atoms, isotopes, and their periodic table representations at phet. Discuss how the bohr model can be used to explain atomic spectra. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. In the nucleus you would show the 12. Describe the arrangement of electrons. The Magnesium Bohr Model.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube The Magnesium Bohr Model 119 rows access detailed info on all elements: Define an energy level in terms of the bohr model. Discuss how the bohr model can be used to explain atomic spectra. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. In the nucleus you would show the 12. Atomic mass, electron configurations, charges, and more. In. The Magnesium Bohr Model.
From mungfali.com
Magnesium Atom Bohr Model The Magnesium Bohr Model In the nucleus you would show the 12. Define an energy level in terms of the bohr model. Atomic mass, electron configurations, charges, and more. Magnesium has 3 energy levels: 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. In this video we'll look at the atomic structure and bohr model. The Magnesium Bohr Model.
From ar.inspiredpencil.com
Bohr Model Of Magnesium The Magnesium Bohr Model Describe the arrangement of electrons using the shell model. The first electron shell of a bohr model holds 2 electrons. Atomic mass, electron configurations, charges, and more. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. 119 rows access detailed info on all elements: In this video we'll look at the. The Magnesium Bohr Model.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 The Magnesium Bohr Model 119 rows access detailed info on all elements: View rotating bohr models for all 118 elements. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. So far, 10 of magnesium's 12 electrons have been used, so only 2 remain. In 1913, the danish physicist niels bohr proposed a model of the. The Magnesium Bohr Model.