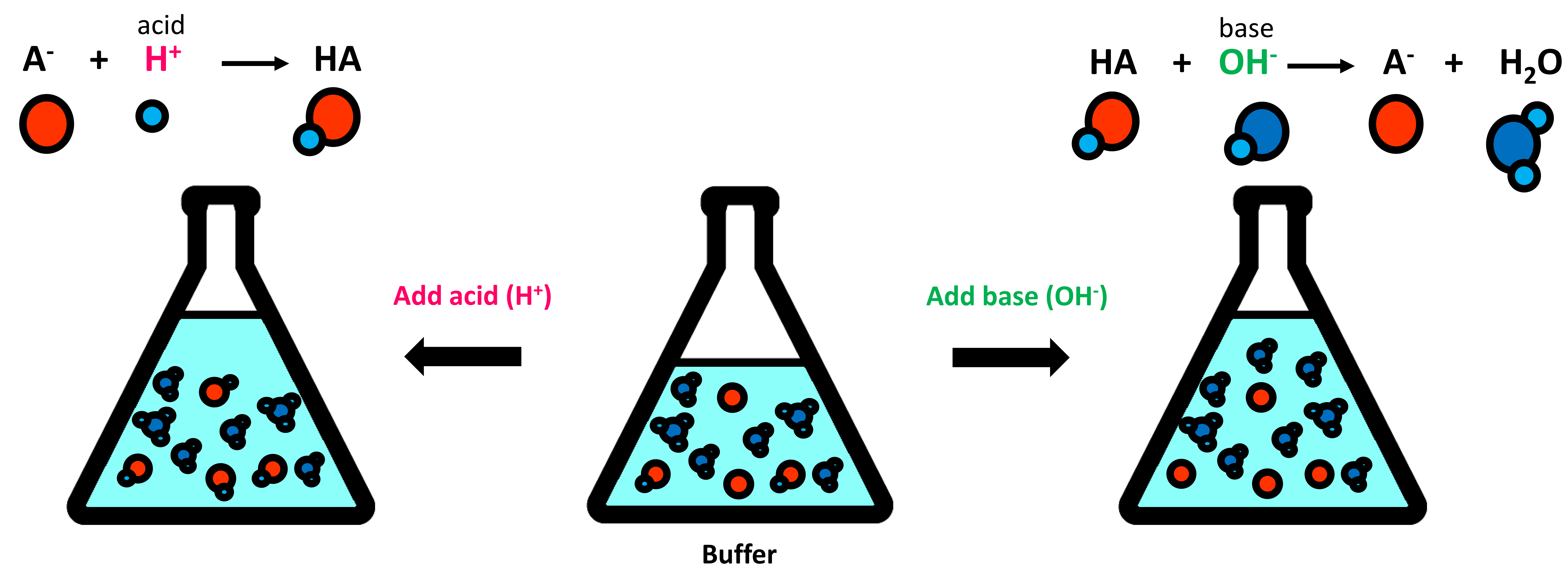
What Are The Biological Importance Of Buffers . The ph of a buffer solution changes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. They act by neutralizing excess hydrogen ions, thereby maintaining the. The aim of this work is to overview the specific effect of ph buffers in biological systems. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Maintenance of biological ph is important. Biological buffers are organic substances that help regulate the ph level in organisms. Biological buffers are compounds that help the body maintain a ph around 7.4. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Four important generalizations about buffers.
from dxouambuf.blob.core.windows.net
A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The aim of this work is to overview the specific effect of ph buffers in biological systems. Four important generalizations about buffers. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are organic substances that help regulate the ph level in organisms. Biological buffers are compounds that help the body maintain a ph around 7.4. The ph of a buffer solution changes.
How To Use A Buffer at Albert Hill blog
What Are The Biological Importance Of Buffers Maintenance of biological ph is important. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. Maintenance of biological ph is important. The ph of a buffer solution changes. The aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are organic substances that help regulate the ph level in organisms. Four important generalizations about buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are compounds that help the body maintain a ph around 7.4. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis.
From joirryihd.blob.core.windows.net
What Are The Buffers In The Body at Frank Stookey blog What Are The Biological Importance Of Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body maintain a ph around 7.4. They act by neutralizing excess hydrogen ions, thereby maintaining the. Maintenance. What Are The Biological Importance Of Buffers.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Are The Biological Importance Of Buffers Biological buffers are compounds that help the body maintain a ph around 7.4. Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Maintenance of biological ph is important. Four important generalizations about buffers. The buffer systems functioning. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT ACID BASE BALANCE PowerPoint Presentation, free download ID2143114 What Are The Biological Importance Of Buffers They act by neutralizing excess hydrogen ions, thereby maintaining the. Four important generalizations about buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are organic substances that help regulate the ph level in organisms. The aim of this work is to overview. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID What Are The Biological Importance Of Buffers The ph of a buffer solution changes. The aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are compounds that help the body maintain a ph around 7.4. Four important generalizations about buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Are The Biological Importance Of Buffers Maintenance of biological ph is important. The ph of a buffer solution changes. They act by neutralizing excess hydrogen ions, thereby maintaining the. Four important generalizations about buffers. The aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are organic substances that help regulate the ph level in organisms. The buffer. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. They act by neutralizing excess hydrogen ions, thereby maintaining the. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a. What Are The Biological Importance Of Buffers.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. The ph of a buffer solution changes. Four important generalizations about buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to. What Are The Biological Importance Of Buffers.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Are The Biological Importance Of Buffers Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Maintenance of biological ph is important. The ph of a buffer solution changes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Four important generalizations about buffers. Biological buffers are organic substances. What Are The Biological Importance Of Buffers.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Are The Biological Importance Of Buffers Maintenance of biological ph is important. Biological buffers are compounds that help the body maintain a ph around 7.4. They act by neutralizing excess hydrogen ions, thereby maintaining the. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The purpose of a buffer in a biological system is to maintain. What Are The Biological Importance Of Buffers.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems What Are The Biological Importance Of Buffers Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The ph of a buffer solution changes. The aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is. What Are The Biological Importance Of Buffers.
From golifescience.com
Buffers What are the Importance of Buffers in Biological system? What Are The Biological Importance Of Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Biological buffers are organic substances that help regulate the ph level in organisms. The ph of a buffer solution changes. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range. What Are The Biological Importance Of Buffers.
From slideplayer.com
Definition; A buffer solution is that solution that resists large What Are The Biological Importance Of Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body maintain a ph around 7.4. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Four important generalizations about buffers. A. What Are The Biological Importance Of Buffers.
From www.slideshare.net
Buffer system What Are The Biological Importance Of Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are organic substances that help regulate the ph level in organisms. The buffer systems functioning in blood. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID What Are The Biological Importance Of Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Biological buffers are organic substances that help regulate the ph level in organisms. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The buffer systems functioning in blood plasma include. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Are The Biological Importance Of Buffers Four important generalizations about buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Biological buffers are organic substances that help regulate the ph level in. What Are The Biological Importance Of Buffers.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Are The Biological Importance Of Buffers They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known. What Are The Biological Importance Of Buffers.
From slideplayer.com
PH and Buffers. ppt download What Are The Biological Importance Of Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are organic substances that help regulate the ph level in organisms. The aim of this work is to overview the specific effect of ph buffers in biological systems. A buffer is composed of an. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are compounds. What Are The Biological Importance Of Buffers.
From slideplayer.com
Biology Concepts & Applications ppt download What Are The Biological Importance Of Buffers Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The ph of a buffer solution changes. Biological buffers are compounds that help the body maintain a ph around 7.4. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions,. What Are The Biological Importance Of Buffers.
From www.youtube.com
Role of buffers in biological system YouTube What Are The Biological Importance Of Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Maintenance of biological ph is important. Biological buffers are organic substances that help regulate the ph level in organisms. Biological buffers are compounds that help the body maintain a ph around 7.4. Buffering is important in living systems as a means of maintaining. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The ph of. What Are The Biological Importance Of Buffers.
From joifezsdx.blob.core.windows.net
Biological Buffers Ppt at Erin Dodds blog What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. The ph of a buffer solution changes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffering is important in living systems as a means of maintaining a fairly. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT The Renal Role in Acid Base Balance PowerPoint Presentation, free What Are The Biological Importance Of Buffers Maintenance of biological ph is important. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The aim of this work is to overview the specific effect of ph buffers in biological systems. Four. What Are The Biological Importance Of Buffers.
From agscientific.com
5 Things to Consider When Choosing Biological Buffers What Are The Biological Importance Of Buffers They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biological buffers are organic substances that help regulate the ph level in organisms. The ph of a buffer solution changes. Four important generalizations about buffers. The buffer systems functioning in. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT 8.3 Bases PowerPoint Presentation, free download ID5917783 What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. The ph of a buffer solution changes. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Are The Biological Importance Of Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Four important generalizations about buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. They act by neutralizing excess hydrogen ions, thereby maintaining the. A buffer is composed. What Are The Biological Importance Of Buffers.
From joipdcnio.blob.core.windows.net
What Is A Buffer Navy at Evelyn Klinger blog What Are The Biological Importance Of Buffers Biological buffers are compounds that help the body maintain a ph around 7.4. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Maintenance of biological ph is important. Biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess hydrogen ions, thereby. What Are The Biological Importance Of Buffers.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Are The Biological Importance Of Buffers The ph of a buffer solution changes. Biological buffers are compounds that help the body maintain a ph around 7.4. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are organic substances that help regulate the ph level in organisms.. What Are The Biological Importance Of Buffers.
From slidetodoc.com
Applications of Aqueous Equilibria Buffers AcidBase Titrations and What Are The Biological Importance Of Buffers Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biological buffers are compounds that help the body maintain a ph around 7.4. They act by neutralizing excess hydrogen ions, thereby maintaining the. Maintenance of biological ph is important. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. What Are The Biological Importance Of Buffers.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and What Are The Biological Importance Of Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are organic substances that. What Are The Biological Importance Of Buffers.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Are The Biological Importance Of Buffers The ph of a buffer solution changes. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are organic substances that help regulate the ph level in organisms. The purpose of a buffer in a biological system is. What Are The Biological Importance Of Buffers.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important What Are The Biological Importance Of Buffers Biological buffers are organic substances that help regulate the ph level in organisms. The ph of a buffer solution changes. The aim of this work is to overview the specific effect of ph buffers in biological systems. Four important generalizations about buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffering is important in living systems as a. What Are The Biological Importance Of Buffers.
From slideplayer.com
Acids, Bases, & pH TSW differentiate between acids and bases, and What Are The Biological Importance Of Buffers The ph of a buffer solution changes. Biological buffers are compounds that help the body maintain a ph around 7.4. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. They act by neutralizing excess hydrogen ions, thereby maintaining the. Maintenance of biological ph is important. The aim of this work. What Are The Biological Importance Of Buffers.
From www.pinterest.com
What are the Buffers and its Importance? This article explains the What Are The Biological Importance Of Buffers Four important generalizations about buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. The aim of this work is to overview the specific effect of ph buffers in biological systems. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The purpose of a buffer in a biological. What Are The Biological Importance Of Buffers.