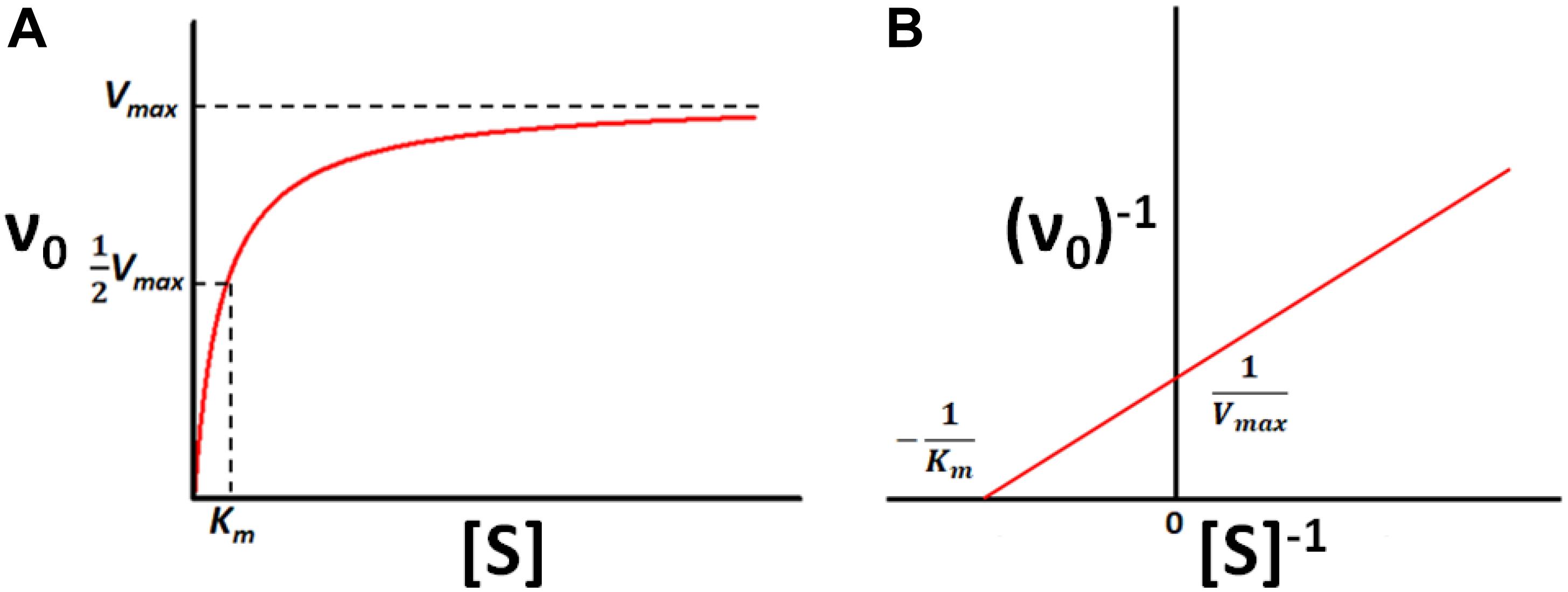
Standard Curve Enzyme Kinetics . Activity (represented in bold italics) can be determined. These values should be used as. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. The enzyme and substrate have to. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. 1 v0 = km + s vms = (km vm)1 s + 1 vm.
from www.frontiersin.org
Activity (represented in bold italics) can be determined. The enzyme and substrate have to. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. These values should be used as. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e.
Frontiers Enzyme by Isothermal Titration Calorimetry
Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. The enzyme and substrate have to. These values should be used as. Activity (represented in bold italics) can be determined. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. 1 v0 = km + s vms = (km vm)1 s + 1 vm.
From www.studocu.com
Tutorial Curves Enzymes 091320 Studocu Standard Curve Enzyme Kinetics The enzyme and substrate have to. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Next, we can. Standard Curve Enzyme Kinetics.
From bio.libretexts.org
6.2 Enzyme Biology LibreTexts Standard Curve Enzyme Kinetics 1 v0 = km + s vms = (km vm)1 s + 1 vm. The enzyme and substrate have to. These values should be used as. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. Background in general, enzyme activity is estimated from spectrophotometric data, by taking. Standard Curve Enzyme Kinetics.
From www.reddit.com
Enzyme Titration Curve pKa Identification chemhelp Standard Curve Enzyme Kinetics 1 v0 = km + s vms = (km vm)1 s + 1 vm. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. The enzyme and substrate have to. Activity (represented in bold italics) can be determined. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the. Standard Curve Enzyme Kinetics.
From www.lecturio.com
Enzyme Concise Medical Knowledge Standard Curve Enzyme Kinetics These values should be used as. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Activity (represented in bold italics) can be determined. The enzyme and substrate have to. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Examining enzyme kinetics is critical for understanding cellular systems and. Standard Curve Enzyme Kinetics.
From lessoncampusunspelt.z13.web.core.windows.net
Enzyme Graph Examples Standard Curve Enzyme Kinetics 1 v0 = km + s vms = (km vm)1 s + 1 vm. The enzyme and substrate have to. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Next, we can. Standard Curve Enzyme Kinetics.
From enzymkinetics.com
Enzyme BestCurvFit Software (EZFit, Perrella), Standard Curve Enzyme Kinetics Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. The enzyme and substrate have to. Activity (represented in bold italics) can be determined. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. These values should be used as. In an enzyme. Standard Curve Enzyme Kinetics.
From www.onlinebiologynotes.com
Rate of enzyme reactions and factor affecting the rate of enzyme Standard Curve Enzyme Kinetics These values should be used as. Activity (represented in bold italics) can be determined. 1 v0 = km + s vms = (km vm)1 s + 1 vm. The enzyme and substrate have to. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Examining enzyme kinetics is critical for understanding cellular systems and. Standard Curve Enzyme Kinetics.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Standard Curve Enzyme Kinetics Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Activity (represented in bold italics) can be determined. These values should be used as. 1 v0 = km + s vms = (km vm)1 s + 1 vm. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. The. Standard Curve Enzyme Kinetics.
From www.studocu.com
Enzyme Data ENZYME DATA (CORRECT version) Standard Standard Curve Enzyme Kinetics Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Activity (represented in bold italics) can be. Standard Curve Enzyme Kinetics.
From www.frontiersin.org
Frontiers Enzyme by Isothermal Titration Calorimetry Standard Curve Enzyme Kinetics The enzyme and substrate have to. These values should be used as. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the. Standard Curve Enzyme Kinetics.
From bio.libretexts.org
3.4 Regulation of Enzyme Activity Biology LibreTexts Standard Curve Enzyme Kinetics Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. The enzyme and substrate have to. These values should be used as. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Examining enzyme kinetics is critical for understanding cellular systems and. Standard Curve Enzyme Kinetics.
From gpatindia.com
Enzyme and Question Answer for NEET, GPAT, GATE, SSC Standard Curve Enzyme Kinetics The enzyme and substrate have to. Activity (represented in bold italics) can be determined. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. These values should be used as. In an enzyme. Standard Curve Enzyme Kinetics.
From www.biomol.com
Guide to Enzyme Unit Definitions and Assay Design Biomol Blog Standard Curve Enzyme Kinetics Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. In an enzyme catalyzed reaction the substrate initially forms. Standard Curve Enzyme Kinetics.
From www.lecturio.com
Enzyme Concise Medical Knowledge Standard Curve Enzyme Kinetics Activity (represented in bold italics) can be determined. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. The enzyme and substrate have to. These values should be used as. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part. Standard Curve Enzyme Kinetics.
From www.researchgate.net
Sigmoidal of PpOTC. The graph shows the saturation curve of Standard Curve Enzyme Kinetics Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Activity (represented in bold italics) can be determined. These values should be used as. 1 v0 = km + s vms = (km vm)1 s + 1 vm. In an enzyme catalyzed reaction the substrate initially forms a. Standard Curve Enzyme Kinetics.
From www.youtube.com
maxresdefault.jpg Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. 1 v0 = km + s vms. Standard Curve Enzyme Kinetics.
From www.slideserve.com
PPT Chapter 6.3 Enzyme PowerPoint Presentation, free Standard Curve Enzyme Kinetics 1 v0 = km + s vms = (km vm)1 s + 1 vm. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting. Standard Curve Enzyme Kinetics.
From teachmephysiology.com
Enzyme Structure Function MichaelisMenten Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. The enzyme and substrate have to. Activity (represented in bold italics) can be determined. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the. Standard Curve Enzyme Kinetics.
From www.slideserve.com
PPT Basic Enzymology PowerPoint Presentation ID364307 Standard Curve Enzyme Kinetics These values should be used as. 1 v0 = km + s vms = (km vm)1 s + 1 vm. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Activity (represented in bold italics) can be determined. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the. Standard Curve Enzyme Kinetics.
From www.graphpad.com
GraphPad Prism 10 Curve Fitting Guide Example Fitting an enzyme Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. The enzyme and substrate have to. These values should be used as. Examining enzyme kinetics is critical for understanding cellular systems and. Standard Curve Enzyme Kinetics.
From www.researchgate.net
Model's precision analysis A) curves of the two enzymes tested Standard Curve Enzyme Kinetics Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. These values should be used as. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear. Standard Curve Enzyme Kinetics.
From chem.libretexts.org
10.2 The Equations of Enzyme Chemistry LibreTexts Standard Curve Enzyme Kinetics The enzyme and substrate have to. These values should be used as. 1 v0 = km + s vms = (km vm)1 s + 1 vm. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of. Standard Curve Enzyme Kinetics.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Standard Curve Enzyme Kinetics Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. The enzyme and substrate have to. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Background in general,. Standard Curve Enzyme Kinetics.
From www.cell.com
Thermodynamic ActivityBased Progress Curve Analysis in Enzyme Standard Curve Enzyme Kinetics 1 v0 = km + s vms = (km vm)1 s + 1 vm. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. Background in general, enzyme activity is estimated from. Standard Curve Enzyme Kinetics.
From ar.inspiredpencil.com
Enzymes Activation Energy Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. These values should be used as. The enzyme and substrate have to. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. Background in general, enzyme activity is estimated from spectrophotometric data,. Standard Curve Enzyme Kinetics.
From studymind.co.uk
Enzymes Rates of Reaction (Alevel Biology) Study Mind Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Activity (represented in bold italics) can be determined. The enzyme and substrate have to. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of. Standard Curve Enzyme Kinetics.
From www.slideserve.com
PPT Enzyme Study the rate of enzyme catalyzed reactions Standard Curve Enzyme Kinetics These values should be used as. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. Examining enzyme kinetics is critical for understanding cellular systems and. Standard Curve Enzyme Kinetics.
From www.researchgate.net
Enzyme (A) Michaelis−Menten curve, and (B) corresponding Standard Curve Enzyme Kinetics Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Next, we can estimate the initial reaction. Standard Curve Enzyme Kinetics.
From plantphys.info
Enzyme Standard Curve Enzyme Kinetics Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. In. Standard Curve Enzyme Kinetics.
From www.researchgate.net
Enzyme of DgcR ac Enzymatic progress curves of wildtype DgcR Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The enzyme and substrate have to. Activity (represented in bold italics) can be determined. Background. Standard Curve Enzyme Kinetics.
From www.researchgate.net
An enzyme graph of the initial velocities of uninhibited Standard Curve Enzyme Kinetics These values should be used as. 1 v0 = km + s vms = (km vm)1 s + 1 vm. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Background in general, enzyme activity is estimated from spectrophotometric data, by taking the slope of the linear part of the progress curve. Next, we. Standard Curve Enzyme Kinetics.
From animalia-life.club
Enzyme Activation Energy Graph Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. These values should be used as. Activity (represented. Standard Curve Enzyme Kinetics.
From wou.edu
Chapter 6 Enzyme Principles and Biotechnological Applications Chemistry Standard Curve Enzyme Kinetics Activity (represented in bold italics) can be determined. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. The enzyme and substrate have to. Examining enzyme kinetics is critical for understanding cellular systems and. Standard Curve Enzyme Kinetics.
From www.researchgate.net
The calibration curve about assay of enzyme activity with pNPG as the Standard Curve Enzyme Kinetics The enzyme and substrate have to. In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. 1 v0 = km + s vms = (km vm)1 s + 1 vm. Background in general, enzyme activity is estimated from spectrophotometric. Standard Curve Enzyme Kinetics.
From enzymkinetics.com
Enzyme BestCurvFit Software (EZFit, Perrella), Standard Curve Enzyme Kinetics In an enzyme catalyzed reaction the substrate initially forms a reversible complex with the enzyme (i.e. Next, we can estimate the initial reaction rate (\(v_0\)) at each substrate concentration by plotting the slope of the first few time. 1 v0 = km + s vms = (km vm)1 s + 1 vm. The enzyme and substrate have to. These values. Standard Curve Enzyme Kinetics.