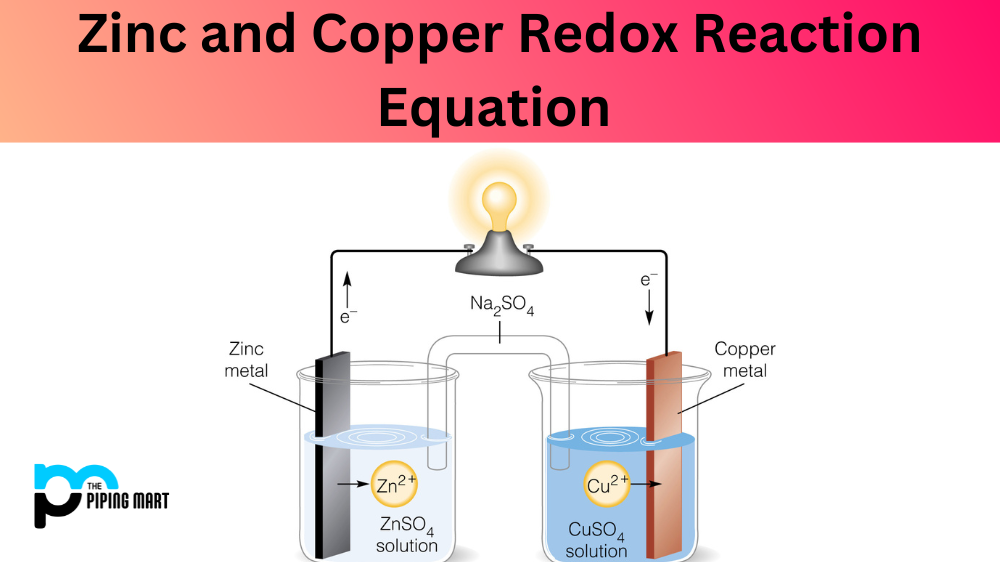
Copper Sulfate Zinc Formula . Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. The lighter group 3a metals (aluminum, galium and. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. Use the chemical equation to explain your. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the.
from blog.thepipingmart.com
Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: The lighter group 3a metals (aluminum, galium and. Use the chemical equation to explain your. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals.
Zinc and Copper Redox Reaction Equation
Copper Sulfate Zinc Formula There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Use the chemical equation to explain your. Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. The lighter group 3a metals (aluminum, galium and. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s).
From www.dreamstime.com
Blue Vitriol, CopperII Sulfate, or Cupric Sulphate, Chemical Formula Copper Sulfate Zinc Formula Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: To balance fe + zn so4 = cu + zn so4. Copper Sulfate Zinc Formula.
From www.fishersci.com
Zinc Sulfate Solution, 0.05M, Honeywell, Quantity 1 L Fisher Scientific Copper Sulfate Zinc Formula Use the chemical equation to explain your. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. The lighter group 3a metals (aluminum, galium and. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. Zinc metal reacts with. Copper Sulfate Zinc Formula.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog Copper Sulfate Zinc Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. The. Copper Sulfate Zinc Formula.
From www.youtube.com
Copper (II) sulfate and zinc reactions YouTube Copper Sulfate Zinc Formula Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Use the chemical equation to explain your. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on. Copper Sulfate Zinc Formula.
From www.youtube.com
H2SO4+Zn=ZnSO4+H2 Balanced EquationSulphuric acid+Zinc=Zinc sulphate Copper Sulfate Zinc Formula The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Use the chemical equation to explain your. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count. Copper Sulfate Zinc Formula.
From www.youtube.com
What happens when a zinc strip is dipped into a copper sulphate Copper Sulfate Zinc Formula There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Use the chemical equation to explain your. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The lighter group 3a metals (aluminum,. Copper Sulfate Zinc Formula.
From dangeloghoposborne.blogspot.com
Molecular Equation for Copper Sulfate Pentahydrate With Heat Copper Sulfate Zinc Formula Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). The lighter group 3a metals (aluminum, galium and. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the.. Copper Sulfate Zinc Formula.
From www.chegg.com
Solved copper(II) sulfate + zinc → zinc sulfate + copper Copper Sulfate Zinc Formula The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. The lighter group 3a metals (aluminum, galium and. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Use the chemical equation to explain your. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4). Copper Sulfate Zinc Formula.
From testbook.com
Copper Sulphate Formula Structure, Preparation, Properties & Uses Copper Sulfate Zinc Formula Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Use. Copper Sulfate Zinc Formula.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Copper Sulfate Zinc Formula To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. Use the chemical equation to explain your. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole. Copper Sulfate Zinc Formula.
From www.dreamstime.com
Zinc Sulfate is a Molecular Chemical Formula. Zinc Infographics. Vector Copper Sulfate Zinc Formula To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. There are three main steps for writing the net ionic equation. Copper Sulfate Zinc Formula.
From www.dreamstime.com
Zinc Sulfate is a Molecular Chemical Formula. Zinc Infographics. Vector Copper Sulfate Zinc Formula Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the. Copper Sulfate Zinc Formula.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0148 Science Copper Sulfate Zinc Formula Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). The chemical equation for the reaction between zinc and copper (ii) sulfate is shown. Copper Sulfate Zinc Formula.
From www.animalia-life.club
Crystallisation Of Copper Sulphate Copper Sulfate Zinc Formula Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Use the chemical equation to explain your. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Cuso4. Copper Sulfate Zinc Formula.
From stock.adobe.com
Molecular formula of copper sulfate. Chemical structure of copper Copper Sulfate Zinc Formula The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. The lighter group 3a metals (aluminum, galium and. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. Use the chemical equation to explain your. Zinc (zn) is more. Copper Sulfate Zinc Formula.
From www.youtube.com
Make Zinc Sulfate, and Copper Metal YouTube Copper Sulfate Zinc Formula Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4). Copper Sulfate Zinc Formula.
From www.alamy.com
Zinc sulfate is a molecular chemical formula. Zinc infographics. Vector Copper Sulfate Zinc Formula Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: To balance fe + zn so4 = cu + zn. Copper Sulfate Zinc Formula.
From www.youtube.com
Copper Sulfate + Zinc YouTube Copper Sulfate Zinc Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. The lighter group 3a metals (aluminum, galium and. The chemical equation for the reaction between zinc and copper (ii). Copper Sulfate Zinc Formula.
From www.youtube.com
How to Write the Formula for Copper (I) sulfate YouTube Copper Sulfate Zinc Formula Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Use the chemical equation to explain your. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each. Copper Sulfate Zinc Formula.
From www.youtube.com
How to Balance Zn + S = ZnS (and Type of Reaction) YouTube Copper Sulfate Zinc Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. To balance fe + zn so4 = cu + zn. Copper Sulfate Zinc Formula.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Copper Sulfate Zinc Formula There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. The lighter group 3a metals (aluminum, galium and. Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. To balance fe + zn so4 = cu. Copper Sulfate Zinc Formula.
From www.dreamstime.com
Zinc Sulfate, Chemical Structure. Skeletal Formula. Stock Vector Copper Sulfate Zinc Formula Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. The lighter group 3a metals (aluminum, galium and. Cuso4 + zn = zn(so4) +. Copper Sulfate Zinc Formula.
From blog.thepipingmart.com
Exploring the Different Reactions of Zinc to Acetic Acid and Copper Copper Sulfate Zinc Formula Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. To balance fe + zn so4 =. Copper Sulfate Zinc Formula.
From www.dreamstime.com
3D Image of Zinc Sulfate Skeletal Formula Stock Illustration Copper Sulfate Zinc Formula The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4). Copper Sulfate Zinc Formula.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Copper Sulfate Zinc Formula The lighter group 3a metals (aluminum, galium and. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. To balance fe + zn so4 = cu + zn so4. Copper Sulfate Zinc Formula.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Copper Sulfate Zinc Formula Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. The. Copper Sulfate Zinc Formula.
From www.youtube.com
How to Write the Formula for Zinc sulfide (ZnS) YouTube Copper Sulfate Zinc Formula To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. The lighter group 3a metals (aluminum, galium and. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc and cadmium (group 2b). Copper Sulfate Zinc Formula.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper Sulfate Zinc Formula To balance fe + zn so4 = cu + zn so4 you'll need to be sure to count all of atoms on each side of the. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4). Copper Sulfate Zinc Formula.
From www.pw.live
Copper Sulfate Formula, Structure, Properties, Uses Copper Sulfate Zinc Formula There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Use the chemical equation to explain your. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zn (s). Copper Sulfate Zinc Formula.
From uwaterloo.ca
Zinc metal in a solution of copper(II) sulfate and sulfuric acid from Copper Sulfate Zinc Formula There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Use the chemical equation to explain your. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso. Copper Sulfate Zinc Formula.
From hxejkrhaj.blob.core.windows.net
Copper 2 Sulfate Zinc Equation at Viola Smith blog Copper Sulfate Zinc Formula Use the chemical equation to explain your. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc metal reacts with copper (ii). Copper Sulfate Zinc Formula.
From www.slideshare.net
Replacement reactions Copper Sulfate Zinc Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. The chemical equation for. Copper Sulfate Zinc Formula.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper Sulfate Zinc Formula Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. The chemical equation for the reaction between zinc and copper (ii) sulfate is shown below. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline. Copper Sulfate Zinc Formula.
From www.flexiprep.com
Chemistry Class 11 NCERT Solutions Chapter 1 Some Basic Concepts of Copper Sulfate Zinc Formula Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous cupric sulfate [cuso 4] and. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). There are three main steps for writing the net ionic equation. Copper Sulfate Zinc Formula.
From cymitquimica.com
Zinc sulfate heptahydrate, EP grade 7WGK8729 CymitQuimica Copper Sulfate Zinc Formula Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Use the chemical equation to explain your. Cuso4 + zn = zn(so4) + cu is a single displacement (substitution) reaction where one mole of aqueous. Copper Sulfate Zinc Formula.