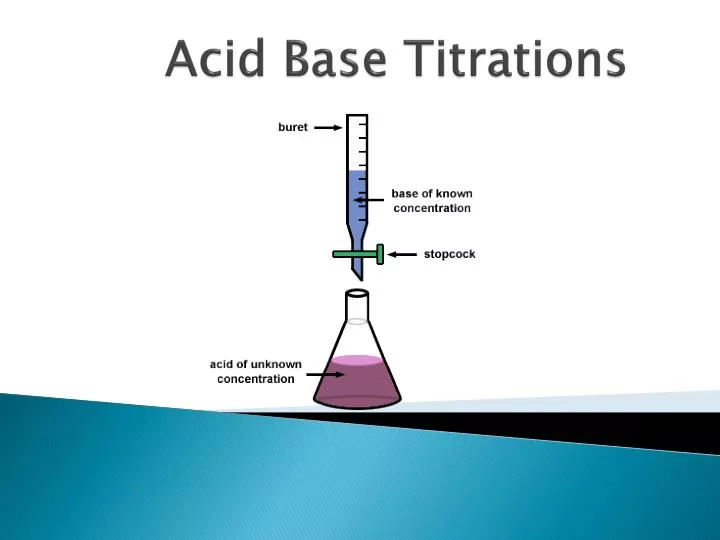
Acid-Base Titration Lab Report Theory . titrating sodium hydroxide with hydrochloric acid. 10 analysis of acids and bases by titration purpose. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the. In this experiment students neutralise sodium. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. In association with nuffield foundation. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution.
from mungfali.com
In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. 10 analysis of acids and bases by titration purpose. In this experiment students neutralise sodium. The analyte (titrand) is the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to.
Acid Base Titration Experiment
Acid-Base Titration Lab Report Theory Be able to determine the k a or k b from ph data associated with the titration of a weak. 10 analysis of acids and bases by titration purpose. The analyte (titrand) is the. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. In this experiment students neutralise sodium. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. titrating sodium hydroxide with hydrochloric acid. In association with nuffield foundation.
From www.vrogue.co
Acid Base Titration Theory Titration Lab Titration St vrogue.co Acid-Base Titration Lab Report Theory a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titrating sodium hydroxide with hydrochloric acid. It is based on the neutralization. 10 analysis of acids and bases by titration purpose. The analyte (titrand) is the. a titration is an experiment where a volume of. Acid-Base Titration Lab Report Theory.
From mungfali.com
Acid Base Titration Lab Acid-Base Titration Lab Report Theory a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. In association with nuffield foundation. a titration is an experiment where. Acid-Base Titration Lab Report Theory.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Acid-Base Titration Lab Report Theory Be able to determine the k a or k b from ph data associated with the titration of a weak. In association with nuffield foundation. The analyte (titrand) is the. In this experiment students neutralise sodium. 10 analysis of acids and bases by titration purpose. a titration is an experiment where a volume of a solution of known. Acid-Base Titration Lab Report Theory.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Acid-Base Titration Lab Report Theory a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment students neutralise sodium. 10 analysis of acids and. Acid-Base Titration Lab Report Theory.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid-Base Titration Lab Report Theory a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. It is based on the neutralization. The analyte (titrand) is the. In this experiment students neutralise sodium. a titration is an experiment where a volume of a solution of known concentration is added to a volume of. Acid-Base Titration Lab Report Theory.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Acid-Base Titration Lab Report Theory In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. 10 analysis of acids and bases by titration purpose. In this experiment students neutralise sodium. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. It is based on the neutralization.. Acid-Base Titration Lab Report Theory.
From childhealthpolicy.vumc.org
😍 Titration of acids and bases lab report. Acid and Base Titrations Lab Acid-Base Titration Lab Report Theory 10 analysis of acids and bases by titration purpose. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. titrating sodium. Acid-Base Titration Lab Report Theory.
From aylinmeowstuart.blogspot.com
Acid Base Titration Experiment Report Acid-Base Titration Lab Report Theory 10 analysis of acids and bases by titration purpose. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. Be able to determine the k a or k b from ph data associated with the titration of a weak. The analyte (titrand) is the.. Acid-Base Titration Lab Report Theory.
From www.vrogue.co
Acid Base Titration Theory Titration Lab Titration St vrogue.co Acid-Base Titration Lab Report Theory It is based on the neutralization. In this experiment students neutralise sodium. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. titrating sodium hydroxide with hydrochloric acid. 10 analysis of acids and bases by titration purpose. Be able to determine the k. Acid-Base Titration Lab Report Theory.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 10 м A. Acid-Base Titration Lab Report Theory titrating sodium hydroxide with hydrochloric acid. In association with nuffield foundation. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. In this experiment students neutralise sodium. 10 analysis of acids and bases by titration purpose. a titration is an analytical procedure. Acid-Base Titration Lab Report Theory.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Acid-Base Titration Lab Report Theory The analyte (titrand) is the. 10 analysis of acids and bases by titration purpose. In this experiment students neutralise sodium. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. In association with nuffield foundation. a titration is a process in which. Acid-Base Titration Lab Report Theory.
From exoqbgfse.blob.core.windows.net
Sample Lab Report On Acid Base Titration at Michelle Hamilton blog Acid-Base Titration Lab Report Theory The analyte (titrand) is the. In association with nuffield foundation. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. It is based on the neutralization. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting. Acid-Base Titration Lab Report Theory.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Acid-Base Titration Lab Report Theory The analyte (titrand) is the. In association with nuffield foundation. In this experiment students neutralise sodium. titrating sodium hydroxide with hydrochloric acid. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Be able to determine the k a or k b from. Acid-Base Titration Lab Report Theory.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Acid-Base Titration Lab Report Theory a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. 10 analysis of acids and bases by. Acid-Base Titration Lab Report Theory.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Acid-Base Titration Lab Report Theory It is based on the neutralization. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Be able to determine the k. Acid-Base Titration Lab Report Theory.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Acid-Base Titration Lab Report Theory 10 analysis of acids and bases by titration purpose. titrating sodium hydroxide with hydrochloric acid. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. In this experiment students neutralise sodium. a titration is a process in which a solution containing a known amount of. Acid-Base Titration Lab Report Theory.
From dxokhzpxh.blob.core.windows.net
Aim Of Acid Base Titration Experiment at Ebony Mooney blog Acid-Base Titration Lab Report Theory titrating sodium hydroxide with hydrochloric acid. Be able to determine the k a or k b from ph data associated with the titration of a weak. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The analyte (titrand) is the. a titration is an experiment. Acid-Base Titration Lab Report Theory.
From www.poshpooch.ca
Acid base titrations lab report. 24/7 College Homework Help. Acid-Base Titration Lab Report Theory The analyte (titrand) is the. It is based on the neutralization. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. 10 analysis of acids and bases by titration purpose. a titration is an experiment where a volume of a solution of known concentration is added. Acid-Base Titration Lab Report Theory.
From dxosvuizs.blob.core.windows.net
AcidBase Titration Lab Report Sample at Ronald McWilliams blog Acid-Base Titration Lab Report Theory Be able to determine the k a or k b from ph data associated with the titration of a weak. titrating sodium hydroxide with hydrochloric acid. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. It is based on the neutralization. The. Acid-Base Titration Lab Report Theory.
From www.studocu.com
Acidbase titration lab report Kayla Elie Madisyn Peoples CHEM 114L 4 Acid-Base Titration Lab Report Theory a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Be able to determine the k a or k b from ph data associated with the titration of a weak. In association with nuffield foundation. The analyte (titrand) is the. titrating sodium hydroxide. Acid-Base Titration Lab Report Theory.
From www.vrogue.co
Acid Base Titration Theory Titration Lab Titration St vrogue.co Acid-Base Titration Lab Report Theory a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. titrating sodium hydroxide with hydrochloric acid. Be able to determine the k a or k b from ph data associated with the titration of a weak. The analyte (titrand) is the. a. Acid-Base Titration Lab Report Theory.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Acid-Base Titration Lab Report Theory a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment students neutralise sodium. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. In association. Acid-Base Titration Lab Report Theory.
From www.scribd.com
Lab Report Acid Base PDF Titration Chemistry Acid-Base Titration Lab Report Theory The analyte (titrand) is the. titrating sodium hydroxide with hydrochloric acid. In association with nuffield foundation. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. Be able to determine the k a or k b from ph data associated with the titration of. Acid-Base Titration Lab Report Theory.
From www.studocu.com
Chem lab 3 AcidBase Titration Lab report AcidBase Titration Lab Acid-Base Titration Lab Report Theory In association with nuffield foundation. 10 analysis of acids and bases by titration purpose. It is based on the neutralization. Be able to determine the k a or k b from ph data associated with the titration of a weak. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it. Acid-Base Titration Lab Report Theory.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Acid-Base Titration Lab Report Theory 10 analysis of acids and bases by titration purpose. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. titrating sodium hydroxide with hydrochloric acid. It is based on the neutralization. Be able to determine the k a or k b from ph. Acid-Base Titration Lab Report Theory.
From dxokxgwqt.blob.core.windows.net
Lab Report On Titration at John Salazar blog Acid-Base Titration Lab Report Theory The analyte (titrand) is the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. It is based on the neutralization. In association. Acid-Base Titration Lab Report Theory.
From www.scribd.com
AcidBase Titration Chemistry Report Acid-Base Titration Lab Report Theory In this experiment students neutralise sodium. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. It is based on the neutralization.. Acid-Base Titration Lab Report Theory.
From www.yumpu.com
Acid base titration lab report.pdf Acid-Base Titration Lab Report Theory a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. It is based on the neutralization. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. Be able to. Acid-Base Titration Lab Report Theory.
From www.studypool.com
SOLUTION Chemistry Lab 1 for Engineering (CHEM 175). AcidBase Acid-Base Titration Lab Report Theory In association with nuffield foundation. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titrating sodium hydroxide with hydrochloric acid. It is based on the neutralization. In this experiment students neutralise sodium. a titration is an experiment where a volume of a solution of known. Acid-Base Titration Lab Report Theory.
From mungfali.com
Acid Base Titration Lab Acid-Base Titration Lab Report Theory In association with nuffield foundation. 10 analysis of acids and bases by titration purpose. The analyte (titrand) is the. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment students neutralise sodium. titrating sodium hydroxide with hydrochloric acid. It. Acid-Base Titration Lab Report Theory.
From www.studeersnel.nl
Acid Base Lab lactic acid titration lab report Acid Base Titrations Acid-Base Titration Lab Report Theory a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. In this experiment students neutralise sodium. Be able to determine the k a or k b from ph data associated with the titration of a weak. It is based on the neutralization. In association with nuffield foundation. . Acid-Base Titration Lab Report Theory.
From presley-study-exams.blogspot.com
Acid Base Titration Lab Report Sheet 33+ Pages Analysis in Doc [2.6mb Acid-Base Titration Lab Report Theory Be able to determine the k a or k b from ph data associated with the titration of a weak. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The analyte (titrand) is the. titrating sodium hydroxide with hydrochloric acid. 10 analysis. Acid-Base Titration Lab Report Theory.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Acid-Base Titration Lab Report Theory In association with nuffield foundation. It is based on the neutralization. The analyte (titrand) is the. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Be able to determine the k a or k b from ph data associated with the titration of. Acid-Base Titration Lab Report Theory.
From www.studypool.com
SOLUTION Chemistry Lab 1 for Engineering (CHEM 175). AcidBase Acid-Base Titration Lab Report Theory In association with nuffield foundation. The analyte (titrand) is the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. 10 analysis of acids and bases by titration purpose. Be able to determine the k a or k b from ph data associated with the titration of. Acid-Base Titration Lab Report Theory.
From mungfali.com
Acid Base Titration Experiment Acid-Base Titration Lab Report Theory a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. titrating sodium hydroxide with hydrochloric acid. The analyte (titrand) is the. 10 analysis of acids and bases by titration purpose. It is based on the neutralization. Be able to determine the k. Acid-Base Titration Lab Report Theory.