Magnesium Nuclear Charge . approximate the effective nuclear charge of magnesium. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. First, compute the overall shielding effect of the electrons orbiting the nucleus. Subtract this value from the nuclear. But looking at magnesium which has 10. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. First we must determine the electron configuration of magnesium to determine the number of. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. to calculate the effective nuclear charge:
from www.alamy.com
First we must determine the electron configuration of magnesium to determine the number of. approximate the effective nuclear charge of magnesium. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. to calculate the effective nuclear charge: charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. Subtract this value from the nuclear. But looking at magnesium which has 10. First, compute the overall shielding effect of the electrons orbiting the nucleus. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled.
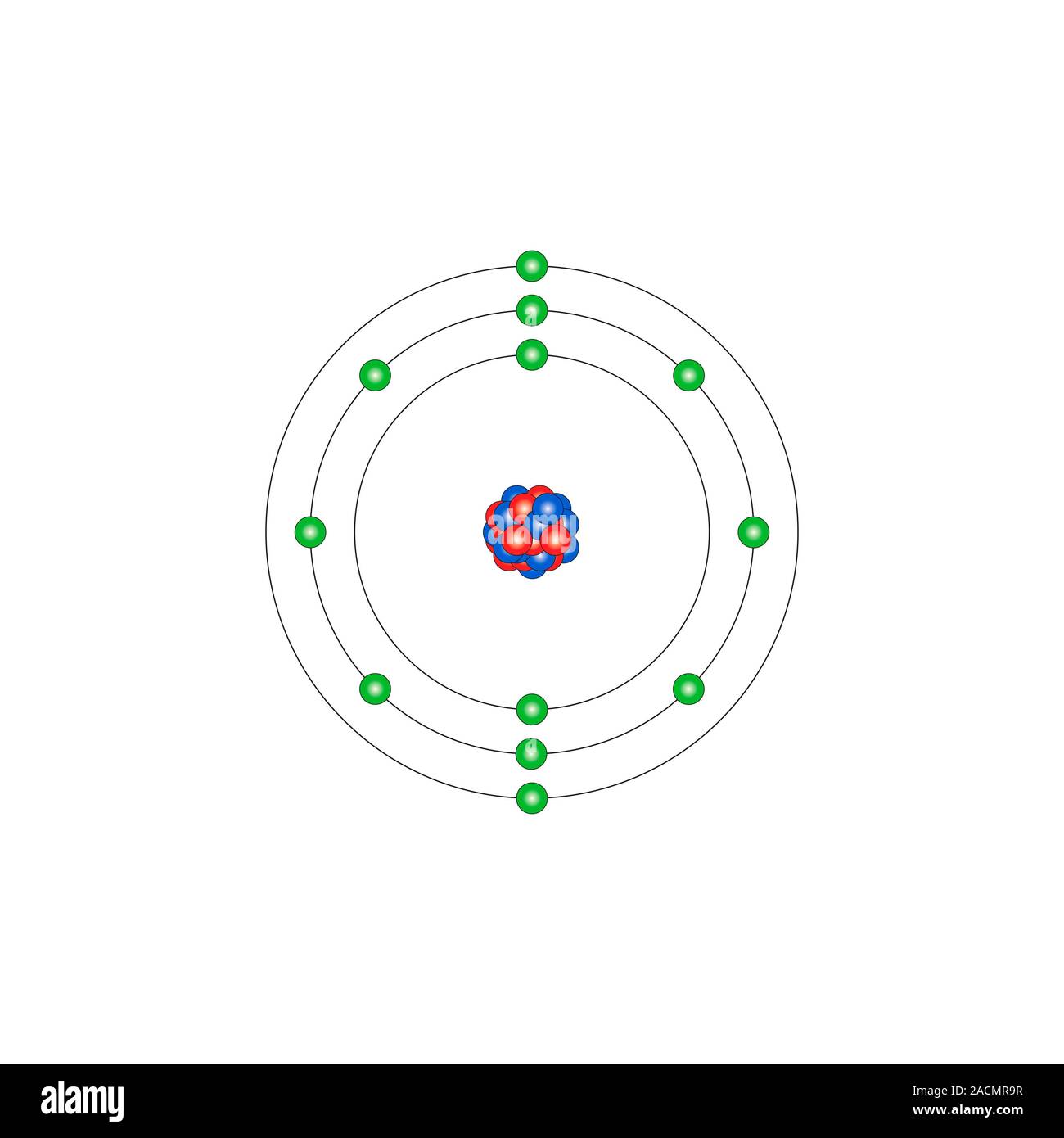
Magnesium (Mg). Diagram of the nuclear composition and electron
Magnesium Nuclear Charge what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. Subtract this value from the nuclear. But looking at magnesium which has 10. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. First we must determine the electron configuration of magnesium to determine the number of. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. First, compute the overall shielding effect of the electrons orbiting the nucleus. to calculate the effective nuclear charge: approximate the effective nuclear charge of magnesium.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2069205 Magnesium Nuclear Charge First, compute the overall shielding effect of the electrons orbiting the nucleus. But looking at magnesium which has 10. Subtract this value from the nuclear. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. to calculate the effective nuclear charge: what is the effective attraction \(z_{eff}\). Magnesium Nuclear Charge.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Nuclear Charge charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium. Magnesium Nuclear Charge.
From www.youtube.com
How To Calculate The Effective Nuclear Charge of an Electron YouTube Magnesium Nuclear Charge First, compute the overall shielding effect of the electrons orbiting the nucleus. But looking at magnesium which has 10. to calculate the effective nuclear charge: Subtract this value from the nuclear. First we must determine the electron configuration of magnesium to determine the number of. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the. Magnesium Nuclear Charge.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Magnesium Nuclear Charge But looking at magnesium which has 10. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. First, compute the overall shielding effect of the. Magnesium Nuclear Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Nuclear Charge But looking at magnesium which has 10. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. to calculate the effective nuclear charge: Subtract this value from the nuclear. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to. Magnesium Nuclear Charge.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Magnesium Nuclear Charge the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. Subtract this value from the nuclear. First, compute the overall shielding effect of the electrons orbiting the nucleus. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the. Magnesium Nuclear Charge.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Nuclear Charge First we must determine the electron configuration of magnesium to determine the number of. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. First, compute the overall shielding effect of the electrons orbiting the nucleus. But looking at magnesium which has 10. approximate the effective nuclear charge of magnesium.. Magnesium Nuclear Charge.
From userdatadorgan.z21.web.core.windows.net
Magnesium Aluminum Phase Diagram Magnesium Nuclear Charge Subtract this value from the nuclear. But looking at magnesium which has 10. First we must determine the electron configuration of magnesium to determine the number of. First, compute the overall shielding effect of the electrons orbiting the nucleus. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout.. Magnesium Nuclear Charge.
From enginelistjuprefecture.z21.web.core.windows.net
Magnesium Atomic Structure Diagram Magnesium Nuclear Charge approximate the effective nuclear charge of magnesium. First we must determine the electron configuration of magnesium to determine the number of. to calculate the effective nuclear charge: Subtract this value from the nuclear. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. the effective nuclear. Magnesium Nuclear Charge.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Nuclear Charge But looking at magnesium which has 10. First, compute the overall shielding effect of the electrons orbiting the nucleus. approximate the effective nuclear charge of magnesium. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. the effective nuclear charge changes relatively little for electrons in the. Magnesium Nuclear Charge.
From www.slideserve.com
PPT Nuclear model of atom PowerPoint Presentation, free download ID Magnesium Nuclear Charge First, compute the overall shielding effect of the electrons orbiting the nucleus. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. First we must determine the electron configuration of magnesium to determine the number of. approximate the effective nuclear charge of magnesium. what. Magnesium Nuclear Charge.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium Nuclear Charge First we must determine the electron configuration of magnesium to determine the number of. to calculate the effective nuclear charge: approximate the effective nuclear charge of magnesium. Subtract this value from the nuclear. First, compute the overall shielding effect of the electrons orbiting the nucleus. charge radii of all magnesium isotopes in the sd shell have been. Magnesium Nuclear Charge.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Magnesium Nuclear Charge the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. approximate the effective nuclear charge of magnesium. But looking at magnesium which has 10. Subtract this value from the nuclear. to calculate the effective nuclear charge: what is the effective attraction \(z_{eff}\) experienced. Magnesium Nuclear Charge.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Nuclear Charge First, compute the overall shielding effect of the electrons orbiting the nucleus. to calculate the effective nuclear charge: First we must determine the electron configuration of magnesium to determine the number of. But looking at magnesium which has 10. approximate the effective nuclear charge of magnesium. Subtract this value from the nuclear. what is the effective attraction. Magnesium Nuclear Charge.
From www.wizeprep.com
Effective Nuclear Charge (Zeff) Wize University Chemistry Textbook Magnesium Nuclear Charge approximate the effective nuclear charge of magnesium. Subtract this value from the nuclear. But looking at magnesium which has 10. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral. Magnesium Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Magnesium Nuclear Charge First, compute the overall shielding effect of the electrons orbiting the nucleus. But looking at magnesium which has 10. First we must determine the electron configuration of magnesium to determine the number of. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. charge radii of all magnesium isotopes in. Magnesium Nuclear Charge.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Nuclear Charge the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. But looking at magnesium which has 10. approximate the effective nuclear charge of magnesium. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout.. Magnesium Nuclear Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Magnesium Nuclear Charge approximate the effective nuclear charge of magnesium. But looking at magnesium which has 10. First, compute the overall shielding effect of the electrons orbiting the nucleus. First we must determine the electron configuration of magnesium to determine the number of. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear. Magnesium Nuclear Charge.
From www.webelements.com
Elements Periodic Table » Magnesium » properties of free atoms Magnesium Nuclear Charge But looking at magnesium which has 10. to calculate the effective nuclear charge: the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. . Magnesium Nuclear Charge.
From www.numerade.com
SOLVED Write the full electronic configurations of both Mg and Mg2 Magnesium Nuclear Charge First we must determine the electron configuration of magnesium to determine the number of. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. . Magnesium Nuclear Charge.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Nuclear Charge First, compute the overall shielding effect of the electrons orbiting the nucleus. to calculate the effective nuclear charge: what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. approximate the effective nuclear charge of magnesium. Subtract this value from the nuclear. But looking at magnesium which has 10. First. Magnesium Nuclear Charge.
From www.alamy.com
Symbol and electron diagram for Magnesium illustration Stock Vector Magnesium Nuclear Charge Subtract this value from the nuclear. First, compute the overall shielding effect of the electrons orbiting the nucleus. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. approximate the effective nuclear charge of magnesium. charge radii of all magnesium isotopes in the sd shell have been measured, revealing. Magnesium Nuclear Charge.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Nuclear Charge what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. First we must determine the electron configuration of magnesium to determine the number of. approximate the effective nuclear charge of magnesium. But looking at magnesium which has 10. First, compute the overall shielding effect of the electrons orbiting the nucleus.. Magnesium Nuclear Charge.
From www.showme.com
Magnesium + Oxygen Ionic Bonding Science, Chemistry ShowMe Magnesium Nuclear Charge Subtract this value from the nuclear. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. approximate the effective nuclear charge of magnesium. . Magnesium Nuclear Charge.
From enginedatanichered.z21.web.core.windows.net
Magnesium Atom Diagram Magnesium Nuclear Charge the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. to calculate the effective nuclear charge: First, compute the overall shielding effect of the electrons orbiting the nucleus. But looking at magnesium which has 10. what is the effective attraction \(z_{eff}\) experienced by the. Magnesium Nuclear Charge.
From www.youtube.com
How To Use Slater's Rule to Estimate The Effective Nuclear Charge YouTube Magnesium Nuclear Charge to calculate the effective nuclear charge: First, compute the overall shielding effect of the electrons orbiting the nucleus. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. approximate the effective nuclear charge of magnesium. Subtract this value from the nuclear. charge radii. Magnesium Nuclear Charge.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Magnesium Nuclear Charge the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. Subtract this value from the nuclear. But looking at magnesium which has 10. First, compute the overall shielding effect of the electrons orbiting the nucleus. approximate the effective nuclear charge of magnesium. charge radii. Magnesium Nuclear Charge.
From cejxrkef.blob.core.windows.net
Magnesium Ion Nuclear Notation at Catherine Lackey blog Magnesium Nuclear Charge approximate the effective nuclear charge of magnesium. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. Subtract this value from the nuclear. the effective nuclear charge changes. Magnesium Nuclear Charge.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Nuclear Charge what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. But looking at magnesium which has 10. the effective nuclear charge changes relatively little for electrons in the outermost,. Magnesium Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Magnesium Nuclear Charge Subtract this value from the nuclear. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. But looking at magnesium which has 10. approximate the effective nuclear charge of magnesium. First, compute the overall shielding effect of the electrons orbiting the nucleus. what is the effective attraction. Magnesium Nuclear Charge.
From ceieufog.blob.core.windows.net
Magnesium Electron Configuration Ground State at Mae Santos blog Magnesium Nuclear Charge First, compute the overall shielding effect of the electrons orbiting the nucleus. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. First we must determine the. Magnesium Nuclear Charge.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Magnesium Nuclear Charge Subtract this value from the nuclear. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. to calculate the effective nuclear charge: approximate. Magnesium Nuclear Charge.
From slideplayer.com
Chemistry I Notes Unit 3 Chapters ppt download Magnesium Nuclear Charge to calculate the effective nuclear charge: what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. First, compute the overall shielding effect of the electrons orbiting the nucleus. approximate the effective nuclear charge of magnesium. Subtract this value from the nuclear. But looking at magnesium which has 10. First. Magnesium Nuclear Charge.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Magnesium Nuclear Charge what is the effective attraction \(z_{eff}\) experienced by the valence electrons in the magnesium anion, the neutral magnesium. the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. First we must determine the electron configuration of magnesium to determine the number of. But looking at. Magnesium Nuclear Charge.
From www.chegg.com
Solved Calculate the effective nuclear charge experienced by Magnesium Nuclear Charge to calculate the effective nuclear charge: the effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. charge radii of all magnesium isotopes in the sd shell have been measured, revealing evolution of the nuclear shape throughout. First, compute the overall shielding effect of the. Magnesium Nuclear Charge.