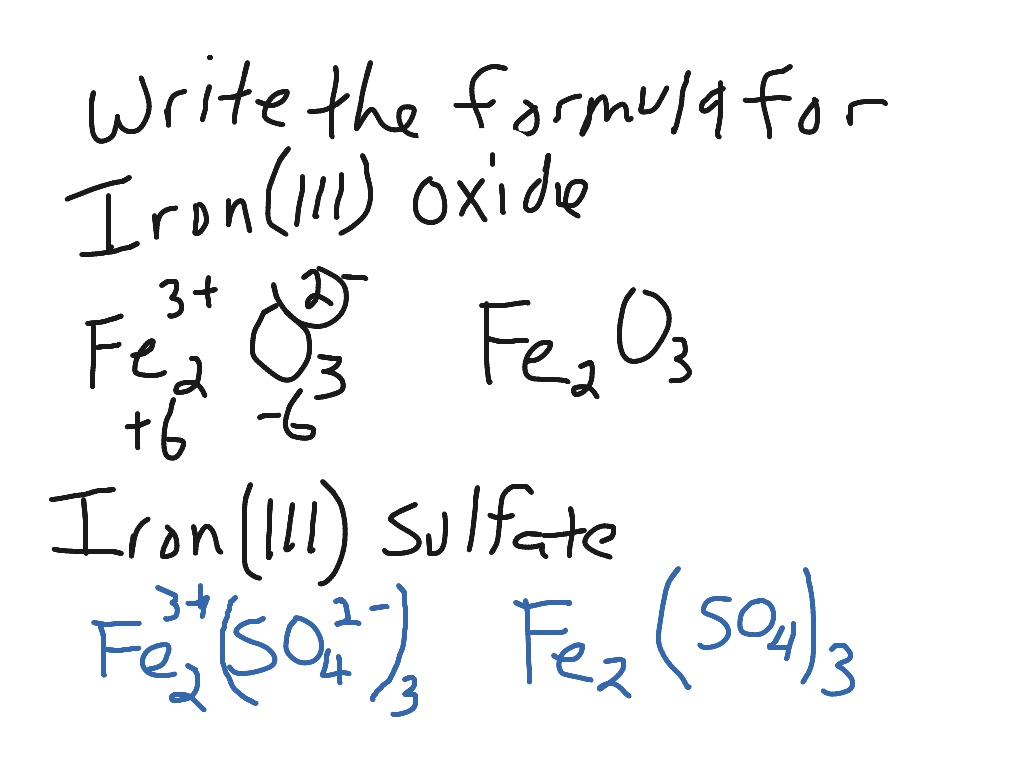How Many Grams Of Iron Iii Oxide Will Be Produced . How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe2o3 are formed when 16.7 g of fe reacts completely. 4 fe + 3 o 2 →2 fe 2 o 3. If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Iron (iii) oxide is formed when iron combines with oxygen in the air. First we need a balanced reaction equation: Question asks about grams of fe 2 o 3;
from www.showme.com
How many grams fe2o3 are formed when 16.7 g of fe reacts completely. Question asks about grams of fe 2 o 3; Iron (iii) oxide is formed when iron combines with oxygen in the air. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. 4 fe + 3 o 2 →2 fe 2 o 3. First we need a balanced reaction equation: If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be.
Write the formula for iron (III) oxide and iron (III) sulfate Chemistry, Science ShowMe
How Many Grams Of Iron Iii Oxide Will Be Produced How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. First we need a balanced reaction equation: Question asks about grams of fe 2 o 3; How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? How many grams fe2o3 are formed when 16.7 g of fe reacts completely. 4 fe + 3 o 2 →2 fe 2 o 3. Iron (iii) oxide is formed when iron combines with oxygen in the air. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in the... Course Hero How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. Iron (iii) oxide is formed when iron combines with oxygen in the. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED 4Iron metal reacts with oxygen to produce iron (III) oxide Write and balance the How Many Grams Of Iron Iii Oxide Will Be Produced If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. First we need a balanced reaction equation: Iron (iii) oxide is formed when iron combines with oxygen in the air. How many grams fe 2 o 3, are produced from the. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.chegg.com
Solved How many moles of iron are needed to produce 2.59 g How Many Grams Of Iron Iii Oxide Will Be Produced If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? First. How Many Grams Of Iron Iii Oxide Will Be Produced.
From slideplayer.com
Calculations Based on Chemical Equations ppt download How Many Grams Of Iron Iii Oxide Will Be Produced If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. 4 fe + 3 o 2. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 How Many Grams Of Iron Iii Oxide Will Be Produced Question asks about grams of fe 2 o 3; How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Iron (iii) oxide is formed when iron combines with oxygen in the air. First we need a balanced reaction equation: How many grams fe2o3 are formed when 16.7 g of fe reacts completely.. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.chegg.com
Solved 3. Iron reacts with oxygen to form iron (III) oxide How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. First we need a balanced reaction equation: Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. If we were to ask. How Many Grams Of Iron Iii Oxide Will Be Produced.
From joigjogsq.blob.core.windows.net
Iron Iii Oxide Negative Ion at Emily Gaines blog How Many Grams Of Iron Iii Oxide Will Be Produced How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? First we need a balanced reaction equation: Question asks about grams of fe 2 o 3; Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. If we were to ask how many grams of elemental iron will be formed. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED 15.0 grams of Iron is allowed to react with air to produce Iron III Oxide. If the How Many Grams Of Iron Iii Oxide Will Be Produced How many grams fe2o3 are formed when 16.7 g of fe reacts completely. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. 4 fe + 3 o 2 →2 fe 2 o 3. How many grams fe 2 o 3,. How Many Grams Of Iron Iii Oxide Will Be Produced.
From brainly.com
1. How many grams of iron (II) oxide can be produced from 3.4 g of iron in this balanced How Many Grams Of Iron Iii Oxide Will Be Produced Iron (iii) oxide is formed when iron combines with oxygen in the air. 4 fe + 3 o 2 →2 fe 2 o 3. Question asks about grams of fe 2 o 3; If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. How many. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED Fe+O2=Fe2O3 If 14.6 g of iron(III) oxide (rust) is produced from a certain amount of How Many Grams Of Iron Iii Oxide Will Be Produced Question asks about grams of fe 2 o 3; How many grams fe2o3 are formed when 16.7 g of fe reacts completely. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Next, since 50.0 grams of iron (iii) oxide is. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to the chemical equation below How Many Grams Of Iron Iii Oxide Will Be Produced Iron (iii) oxide is formed when iron combines with oxygen in the air. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. From the equation, we can see. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon to give iron and carbon Fe2O3 + 3C → 2Fe + 3CO How How Many Grams Of Iron Iii Oxide Will Be Produced If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Question asks about grams of fe. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.chegg.com
Solved Consider the balanced chemical reaction below. What How Many Grams Of Iron Iii Oxide Will Be Produced If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. Question asks about grams of fe 2 o 3; From the equation, we can see that 4 moles of iron react with 3. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVEDTo convert 1 mol of iron(III) oxide to its elements requires 196.5 kcal Fe2O3(s)+196.5 How Many Grams Of Iron Iii Oxide Will Be Produced First we need a balanced reaction equation: If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe 2 o 3, are produced from the complete. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED If 7.00 L of water vapor at 50.2 °C and 0.121 atm reacts with excess iron, how many How Many Grams Of Iron Iii Oxide Will Be Produced Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe2o3 are formed when 16.7 g of fe reacts completely. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. 4 fe + 3 o 2 →2 fe 2 o. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.solutionspile.com
[Solved] Under certain conditions, the substances iron and How Many Grams Of Iron Iii Oxide Will Be Produced First we need a balanced reaction equation: If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. Next, since 50.0. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube How Many Grams Of Iron Iii Oxide Will Be Produced Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. Question asks about grams of. How Many Grams Of Iron Iii Oxide Will Be Produced.
From en.wikipedia.org
Iron(III) oxide Wikipedia How Many Grams Of Iron Iii Oxide Will Be Produced First we need a balanced reaction equation: Iron (iii) oxide is formed when iron combines with oxygen in the air. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 6.00 l of water vapor at 50.2 degrees celcius. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The balanced chemical equation How Many Grams Of Iron Iii Oxide Will Be Produced First we need a balanced reaction equation: How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. Question asks about grams of fe 2 o 3; How. How Many Grams Of Iron Iii Oxide Will Be Produced.
From brainly.com
How many grams of iron (III) oxide are produced when 38 grams of beryllium oxide react with iron How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. Question asks about grams of fe 2 o 3; How many grams. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.slideserve.com
PPT 29 When 84.8 g of iron (III) oxide reacts with excess of carbon monoxide, iron is How Many Grams Of Iron Iii Oxide Will Be Produced From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? 4 fe + 3 o 2 →2 fe 2 o 3. Next, since 50.0 grams of iron. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Chemistry, Science ShowMe How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. Iron (iii) oxide is formed when iron combines with oxygen in the air. How many grams fe2o3 are formed when 16.7 g of fe reacts completely. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii). How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.chegg.com
Solved Name ID A d. 135 g 19.) How many grams of iron(III) How Many Grams Of Iron Iii Oxide Will Be Produced If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. From the equation, we can see. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube How Many Grams Of Iron Iii Oxide Will Be Produced Question asks about grams of fe 2 o 3; Iron (iii) oxide is formed when iron combines with oxygen in the air. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the. How many grams fe 2 o 3, are produced. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.chegg.com
Solved According to the following reaction, how many grams How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0. How Many Grams Of Iron Iii Oxide Will Be Produced.
From slideplayer.com
Chapter 9 Stoichiometry part I ppt download How Many Grams Of Iron Iii Oxide Will Be Produced First we need a balanced reaction equation: Iron (iii) oxide is formed when iron combines with oxygen in the air. 4 fe + 3 o 2 →2 fe 2 o 3. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. Question asks about grams. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses How Many Grams Of Iron Iii Oxide Will Be Produced Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce 2 moles of iron (iii) oxide. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If we were to ask. How Many Grams Of Iron Iii Oxide Will Be Produced.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses How Many Grams Of Iron Iii Oxide Will Be Produced Question asks about grams of fe 2 o 3; 4 fe + 3 o 2 →2 fe 2 o 3. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii). How Many Grams Of Iron Iii Oxide Will Be Produced.
From slideplayer.com
Calculations Based on Chemical Equations ppt download How Many Grams Of Iron Iii Oxide Will Be Produced If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. How many grams fe2o3 are formed when 16.7 g of fe reacts completely. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a precursor of rust) Use How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. From the equation, we can see that 4 moles of iron react with 3 moles of oxygen to produce. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.slideserve.com
PPT Chapter 6 Chemical Composition Counting Atoms PowerPoint Presentation ID4011081 How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. How many grams fe2o3 are formed when 16.7 g of fe reacts completely. First we need a balanced reaction equation: If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use the.. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
SOLVED Determine the mass of Iron (III) oxide (Fe2O3) produced in the reaction of How Many Grams Of Iron Iii Oxide Will Be Produced Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. How many grams fe2o3 are formed when 16.7 g of fe reacts completely. Iron (iii) oxide is formed when iron combines with oxygen in the air. If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii). How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.youtube.com
Gas stoichiometry of iron(III) hydroxide YouTube How Many Grams Of Iron Iii Oxide Will Be Produced If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. First we need a balanced reaction equation: If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii) oxide, we would simply use. How Many Grams Of Iron Iii Oxide Will Be Produced.
From www.numerade.com
A 56g sample of iron reacts with 24 g of oxygen to form how many grams of iron oxide? Numerade How Many Grams Of Iron Iii Oxide Will Be Produced If 6.00 l of water vapor at 50.2 degrees celcius and 0.121 atm reacts with excess iron, how many grams of iron(iii) oxide will be. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. If we were to ask how many grams of elemental iron will be formed by the reduction of 1.0 grams of iron (iii). How Many Grams Of Iron Iii Oxide Will Be Produced.
From slidetodoc.com
STOICHIOMETRY Calculations Based on Chemical Equations Iron III How Many Grams Of Iron Iii Oxide Will Be Produced 4 fe + 3 o 2 →2 fe 2 o 3. Next, since 50.0 grams of iron (iii) oxide is only 78.7% of. Question asks about grams of fe 2 o 3; How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Iron (iii) oxide is formed when iron combines with oxygen. How Many Grams Of Iron Iii Oxide Will Be Produced.