Indicator For Calcium Titration . Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. It contains three ionizable protons and we will represent it by the formula. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. The specific indicator used is eriochrome black t.
from www.studocu.com
• record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. The specific indicator used is eriochrome black t. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. It contains three ionizable protons and we will represent it by the formula. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution.
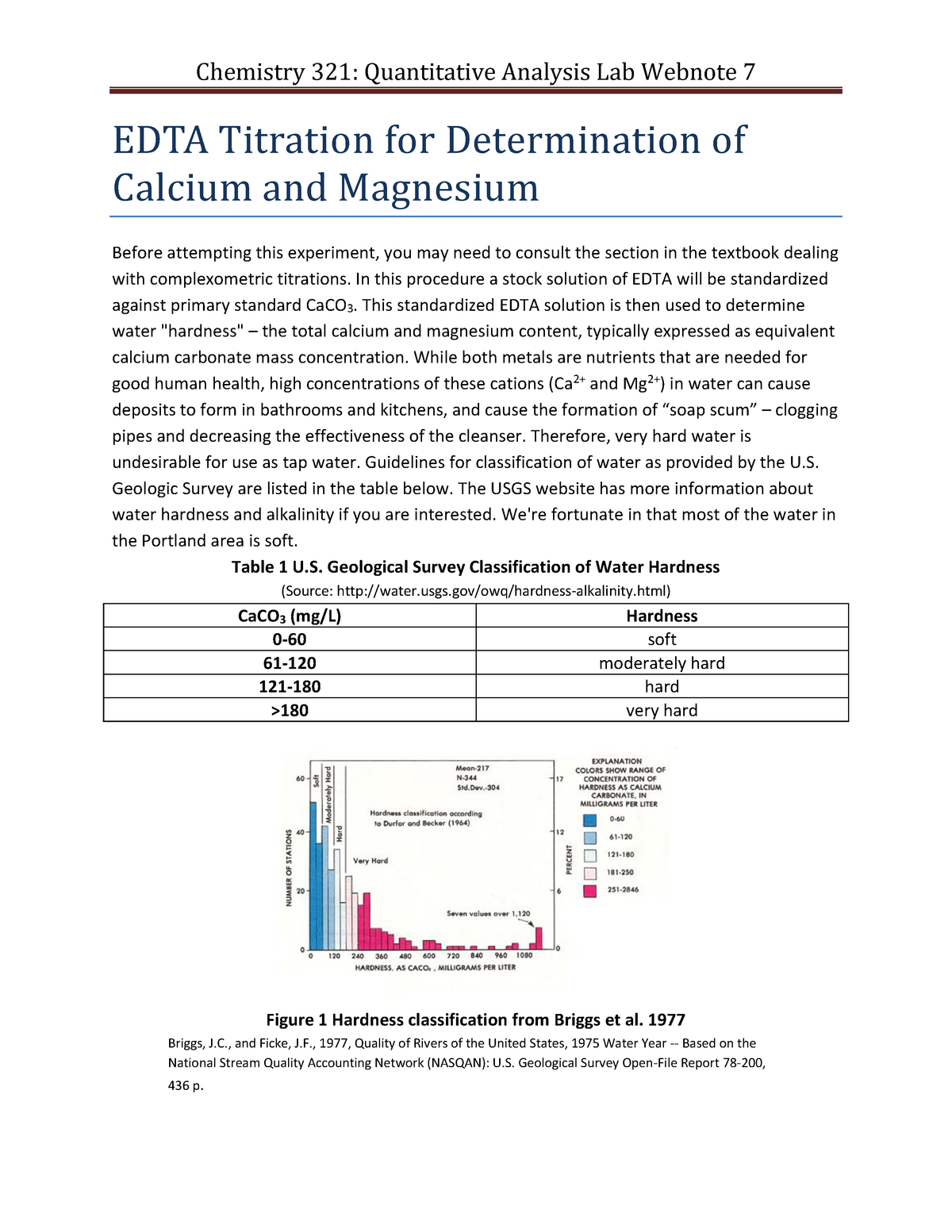
EDTA Titration for Determination of calcium and magnesium In this procedure a stock solution
Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. The specific indicator used is eriochrome black t. It contains three ionizable protons and we will represent it by the formula. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration.
From andor.oxinst.com
How Does Calcium Imaging Work Calcium Indicators Oxford Instruments Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. It contains three ionizable protons and we will represent it by the formula. The specific indicator used is eriochrome black t. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. To. Indicator For Calcium Titration.
From exocowbmz.blob.core.windows.net
Indicator Used In Calcium Titration at Hattie Murphy blog Indicator For Calcium Titration To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. The specific indicator used is eriochrome black t. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. • record the molecular formulas and molar masses of calcium. Indicator For Calcium Titration.
From www.youtube.com
Calcium and Magnesium ion concentration determination with EDTA titration YouTube Indicator For Calcium Titration Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. The specific indicator used is eriochrome black t. • record the molecular formulas and molar masses of calcium. Indicator For Calcium Titration.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Indicator For Calcium Titration It contains three ionizable protons and we will represent it by the formula. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. The specific indicator used is eriochrome black t. To carry out cation titrations using edta,. Indicator For Calcium Titration.
From www.studypool.com
SOLUTION Aim to calculate the solubility of calcium hydroxide ca oh 2 by titration with Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. It contains three ionizable protons and we will represent it by the formula. The specific indicator used is eriochrome black t. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. Since both edta and ca2+ are both. Indicator For Calcium Titration.
From pubs.acs.org
Fluorescent and Bioluminescent Calcium Indicators with Tuneable Colors and Affinities Journal Indicator For Calcium Titration To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. The specific indicator used is eriochrome black t. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To carry out cation titrations using edta, it is necessary. Indicator For Calcium Titration.
From www.researchgate.net
(PDF) New indicator for titration of calcium and total calcium plus magnesium with Indicator For Calcium Titration To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. It contains three ionizable protons and we will represent it by the formula. The specific indicator used is eriochrome black. Indicator For Calcium Titration.
From www.youtube.com
How to Perform the Determination of Ca and Mg in Milk Samples and Calculations YouTube Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. It contains three ionizable protons and we will represent it by the formula. Since both edta and ca2+ are both colorless, it. Indicator For Calcium Titration.
From www.scribd.com
Determination of Calcium Ion Concentration in Water Samples Using EDTA Titration and Calcon Indicator For Calcium Titration To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. The specific indicator used is eriochrome black t. It contains three ionizable protons and we will represent it by the formula. Since both edta and ca2+ are both colorless, it is necessary to use a rather special. Indicator For Calcium Titration.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. It contains three ionizable protons and we will represent it by the formula. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since both edta and ca2+ are both colorless, it. Indicator For Calcium Titration.
From aquaspex.com.au
Calcium Indicator Indicator For Calcium Titration To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To accomplish this displacement titration, a small amount of mg2+ will be. Indicator For Calcium Titration.
From www.studypool.com
SOLUTION Aim to calculate the solubility of calcium hydroxide ca oh 2 by titration with Indicator For Calcium Titration To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. It contains three ionizable protons and we will represent it by the formula. The specific indicator used is eriochrome black t. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To. Indicator For Calcium Titration.
From slidetodoc.com
EDTA Titrations Chelation in Biochemistry Chelating ligands can Indicator For Calcium Titration The specific indicator used is eriochrome black t. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. To accomplish this displacement. Indicator For Calcium Titration.
From www.researchgate.net
Titration procedure to achieve optimal calcium indicator/opsin... Download Scientific Diagram Indicator For Calcium Titration Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. The specific indicator used is eriochrome black t. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. It contains three ionizable protons and we will represent it. Indicator For Calcium Titration.
From www.researchgate.net
Free calcium profiles of titration experiments using four titanium... Download Scientific Diagram Indicator For Calcium Titration Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. The specific indicator used is eriochrome black t. • record the molecular formulas and molar masses of calcium. Indicator For Calcium Titration.
From dokumen.tips
(PDF) New indicator for the complexometric titration of calcium with EDTA DOKUMEN.TIPS Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point. Indicator For Calcium Titration.
From brainly.com
The indicator in a titration changed color after 0.005 mol of calcium hydroxide were added to to Indicator For Calcium Titration It contains three ionizable protons and we will represent it by the formula. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. The specific indicator used is eriochrome black t. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To. Indicator For Calcium Titration.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Indicator For Calcium Titration The specific indicator used is eriochrome black t. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. It contains three ionizable. Indicator For Calcium Titration.
From www.researchgate.net
Schematic illustration of ion selective nanospheres as chelators and... Download Scientific Indicator For Calcium Titration The specific indicator used is eriochrome black t. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since both edta and ca2+ are both colorless, it is necessary to use a. Indicator For Calcium Titration.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Indicator For Calcium Titration To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. The specific indicator used is eriochrome black t. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point. Indicator For Calcium Titration.
From www.slideshare.net
P h indicators Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. The specific indicator used is eriochrome black t. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. It contains three ionizable protons and we will represent it by the formula. Since both edta and ca2+ are both. Indicator For Calcium Titration.
From www.studocu.com
EDTA Titration for Determination of calcium and magnesium In this procedure a stock solution Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. The specific indicator used is eriochrome black t. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the. Indicator For Calcium Titration.
From chemistry.stackexchange.com
analytical chemistry What happens if don't use MgCl₂ in buffer solution in Ca²⁺/Mg²⁺ titration Indicator For Calcium Titration To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. The specific indicator used is eriochrome black t. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. • record the molecular. Indicator For Calcium Titration.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Indicator For Calcium Titration To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. The specific indicator used is eriochrome black t. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since both edta and ca2+ are both colorless, it is necessary to. Indicator For Calcium Titration.
From exocowbmz.blob.core.windows.net
Indicator Used In Calcium Titration at Hattie Murphy blog Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. It contains three ionizable protons and we will represent it by the formula. The specific indicator used is eriochrome black t. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. To. Indicator For Calcium Titration.
From mungfali.com
EDTA Titration Curve Indicator For Calcium Titration To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the. Indicator For Calcium Titration.
From joivkirnp.blob.core.windows.net
What Is An Indicator And What Are They Used For at Lucille Young blog Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. It contains three ionizable protons and we will represent it by the formula. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To accomplish this displacement titration, a small. Indicator For Calcium Titration.
From www.researchgate.net
Calibration plots determined individually for pH indicator, calcium and... Download Scientific Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. It contains three ionizable protons and we will represent it by the formula. The specific indicator used is eriochrome black t. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. To carry out cation titrations using edta,. Indicator For Calcium Titration.
From vdocuments.mx
Als neuen Indicator für die komplexometrische Titration von Calcium und Magnesium [PDF Document] Indicator For Calcium Titration Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. The specific indicator used is eriochrome black t. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. • record the molecular. Indicator For Calcium Titration.
From lulelaboratory.blogspot.com
Lu Le Laboratory The Determination of Calcium by Displacement Titration Lu Le Laboratory Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. It contains three ionizable protons and we will represent it by the formula. To accomplish this displacement titration, a small. Indicator For Calcium Titration.
From www.lazada.com.my
Chromium black T indicator solution Yilai chrome agent 5g/L water hardness detection calcium ion Indicator For Calcium Titration It contains three ionizable protons and we will represent it by the formula. The specific indicator used is eriochrome black t. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. Since. Indicator For Calcium Titration.
From www.researchgate.net
Calcium ion titration of the rate of inactivation at 60 C. Halflife... Download Scientific Indicator For Calcium Titration • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point. Indicator For Calcium Titration.
From www.mdpi.com
Molecules Free FullText Determination of Calcium in Meat Products by Automatic Titration Indicator For Calcium Titration To carry out cation titrations using edta, it is necessary to use an indicator to determine when the equivalence point has been reached. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. It contains three ionizable protons. Indicator For Calcium Titration.
From studylib.net
Determination of Calcium by Titration with EDTA Indicator For Calcium Titration To accomplish this displacement titration, a small amount of mg2+ will be mixed with the edta solution. It contains three ionizable protons and we will represent it by the formula. Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. • record the molecular formulas. Indicator For Calcium Titration.
From www.gopracticals.com
To Determine total hardness of Water sample in terms of Caco3 by EDTA Titration method using Indicator For Calcium Titration Since both edta and ca2+ are both colorless, it is necessary to use a rather special indicator to detect the end point of the titration. • record the molecular formulas and molar masses of calcium carbonate and ethylenediamminetetracetic acid, (disodium. The specific indicator used is eriochrome black t. To accomplish this displacement titration, a small amount of mg2+ will be. Indicator For Calcium Titration.