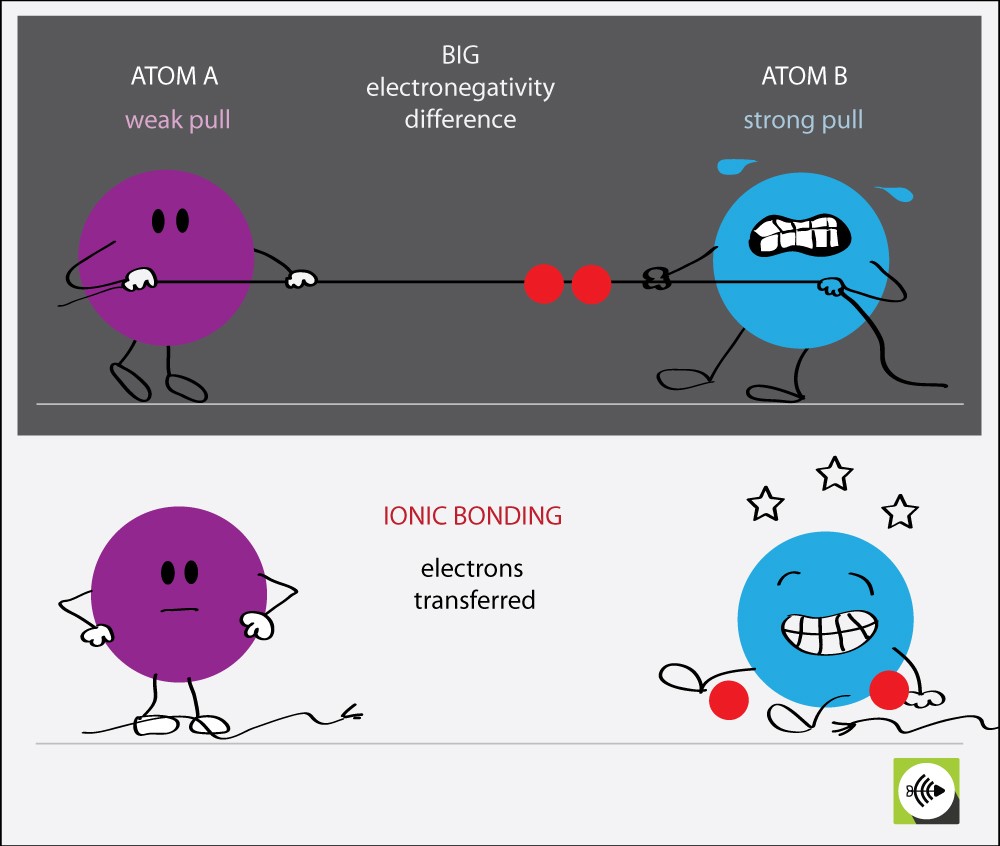
Calcium And Chlorine Electronegativity Difference . 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. It can also be used to predict if the resulting molecule will be polar or. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. It is an extremely reactive element and a strong oxidising agent: The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,.
from surfguppy.com
The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. It is an extremely reactive element and a strong oxidising agent: It can also be used to predict if the resulting molecule will be polar or. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes.
Electronegativity Bond Scale Surfguppy Chemistry made easy visual
Calcium And Chlorine Electronegativity Difference 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. It can also be used to predict if the resulting molecule will be polar or. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. It is an extremely reactive element and a strong oxidising agent: The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 119 rows electronegativity is used to predict whether. Calcium And Chlorine Electronegativity Difference.
From www.learnatnoon.com
Which Halogen Has The Lowest Electronegativity? Noon Academy Calcium And Chlorine Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. It is an extremely reactive element and a strong oxidising agent: The two chlorine atoms share the pair of electrons in the single covalent bond equally, and. Calcium And Chlorine Electronegativity Difference.
From www.nagwa.com
Question Video Identifying Which Silicon Electronegativity Comparison Calcium And Chlorine Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The bond polarity between two atoms. Calcium And Chlorine Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Calcium And Chlorine Electronegativity Difference 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. It can also be used to predict. Calcium And Chlorine Electronegativity Difference.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Calcium And Chlorine Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The two chlorine atoms share the pair of. Calcium And Chlorine Electronegativity Difference.
From www.numerade.com
SOLVED PLEASE HELP 15 POINTS AND MARKED BRAINLIST... Use the chart to Calcium And Chlorine Electronegativity Difference Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. 103 rows this is. Calcium And Chlorine Electronegativity Difference.
From socratic.org
Which Chloride should have the greatest covalent character? Socratic Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 103 rows this is especially problematic for francium, which by relativistic calculations can be. Calcium And Chlorine Electronegativity Difference.
From www.chegg.com
Solved Compound electronegativity difference? ammonia water Calcium And Chlorine Electronegativity Difference 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. It can also be used to predict if the resulting molecule will be polar or. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The electronegativity calculator allows you to calculate. Calcium And Chlorine Electronegativity Difference.
From studyafrikander.z13.web.core.windows.net
How To Determine Electronegativity Calcium And Chlorine Electronegativity Difference The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. It can also be used. Calcium And Chlorine Electronegativity Difference.
From bio.libretexts.org
W2017_Lecture_02_reading Biology LibreTexts Calcium And Chlorine Electronegativity Difference Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the resulting molecule will be polar or. The two chlorine atoms share the pair of electrons in the single covalent. Calcium And Chlorine Electronegativity Difference.
From www.gauthmath.com
Solved 7) For each bond given, determine the bond type and rank in Calcium And Chlorine Electronegativity Difference 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. 119 rows electronegativity is used to predict. Calcium And Chlorine Electronegativity Difference.
From brainly.ph
Answer That one answer Brainly.ph Calcium And Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. It can also be used to predict if. Calcium And Chlorine Electronegativity Difference.
From slideplayer.com
CHEMICAL BONDING. ppt download Calcium And Chlorine Electronegativity Difference The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is. Calcium And Chlorine Electronegativity Difference.
From klarebergsskolan9b.blogspot.com
NO/Teknik 9B Calcium And Chlorine Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows this is especially problematic for francium,. Calcium And Chlorine Electronegativity Difference.
From www.questionai.com
the chart shows the electroniegativity values as Calcium And Chlorine Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. It is an extremely. Calcium And Chlorine Electronegativity Difference.
From www.youtube.com
Electronegativity Scales of Electronegativity Pauling and Mulliken Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the. Calcium And Chlorine Electronegativity Difference.
From material-properties.org
Sodium and Chlorine Comparison Properties Material Properties Calcium And Chlorine Electronegativity Difference 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. It is an extremely. Calcium And Chlorine Electronegativity Difference.
From fr.dreamstime.com
Table Périodique D'Electronegativity Illustration Stock Illustration Calcium And Chlorine Electronegativity Difference Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The two chlorine atoms share the pair. Calcium And Chlorine Electronegativity Difference.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy visual Calcium And Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. It is an extremely. Calcium And Chlorine Electronegativity Difference.
From www.differencebetween.com
Difference Between Ionic and Covalent Bonds Compare the Difference Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Chlorine has 2 stable naturally occuring isotopes while calcium has 5. Calcium And Chlorine Electronegativity Difference.
From reviewhomedecor.co
Second Ionization Energy Periodic Table Trend Review Home Decor Calcium And Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. The bond polarity between. Calcium And Chlorine Electronegativity Difference.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Calcium And Chlorine Electronegativity Difference The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. It can also be used to predict if the resulting molecule will be polar or. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. The electronegativity calculator allows. Calcium And Chlorine Electronegativity Difference.
From aphid4w2lessonlearning.z13.web.core.windows.net
Polarity Of Bonds Chart Calcium And Chlorine Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. 119. Calcium And Chlorine Electronegativity Difference.
From askfilo.com
3. Although chlorine has same electronegativity as nitrogen but the forme.. Calcium And Chlorine Electronegativity Difference Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. It is an extremely reactive element and a strong oxidising agent: The two chlorine atoms share the pair of electrons in the single covalent. Calcium And Chlorine Electronegativity Difference.
From klavljohb.blob.core.windows.net
Potassium Chlorine Electronegativity Difference at Alvin Hughes blog Calcium And Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. It is an extremely reactive element and a strong oxidising agent: It can also be used to predict if. Calcium And Chlorine Electronegativity Difference.
From www.pinterest.com
Printable Periodic Table PDF Printable Periodic Table Calcium And Chlorine Electronegativity Difference Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. It is an extremely reactive element and a strong oxidising agent: It can also be used to predict if the resulting molecule will be polar or. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Calcium And Chlorine Electronegativity Difference.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties Calcium And Chlorine Electronegativity Difference Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The electronegativity calculator allows you to calculate. Calcium And Chlorine Electronegativity Difference.
From slideplayer.com
CHEMICAL BONDING. ppt download Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. It can also be used to predict if the resulting molecule. Calcium And Chlorine Electronegativity Difference.
From socratic.org
What can the electronegativity difference between the atoms in a Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. It can also be used to predict if the resulting molecule will be polar or. The electronegativity calculator allows you to calculate the type of. Calcium And Chlorine Electronegativity Difference.
From slideplayer.com
Chemical Bonding. ppt download Calcium And Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium,. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Calcium And Chlorine Electronegativity Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Calcium And Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. 103 rows this is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative. Calcium And Chlorine Electronegativity Difference.
From klavljohb.blob.core.windows.net
Potassium Chlorine Electronegativity Difference at Alvin Hughes blog Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the. Calcium And Chlorine Electronegativity Difference.
From excelcharts.z13.web.core.windows.net
Electronegativity Chart Printable periodic table of the elements Calcium And Chlorine Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The two chlorine atoms share the pair of electrons in. Calcium And Chlorine Electronegativity Difference.
From www.transtutors.com
(Get Answer) Calcium Has Two Valence Electrons And An Calcium And Chlorine Electronegativity Difference It is an extremely reactive element and a strong oxidising agent: The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\) molecule is. 119 rows electronegativity is used to predict whether. Calcium And Chlorine Electronegativity Difference.
From thonggiocongnghiep.com
What Is The Electronegativity Difference Of Na And Br A Chemical Insight Calcium And Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the resulting molecule will be polar or. Chlorine has 2 stable naturally occuring isotopes while calcium has 5 stable naturally occuring isotopes. The bond polarity between two atoms can be estimated if you know the. Calcium And Chlorine Electronegativity Difference.