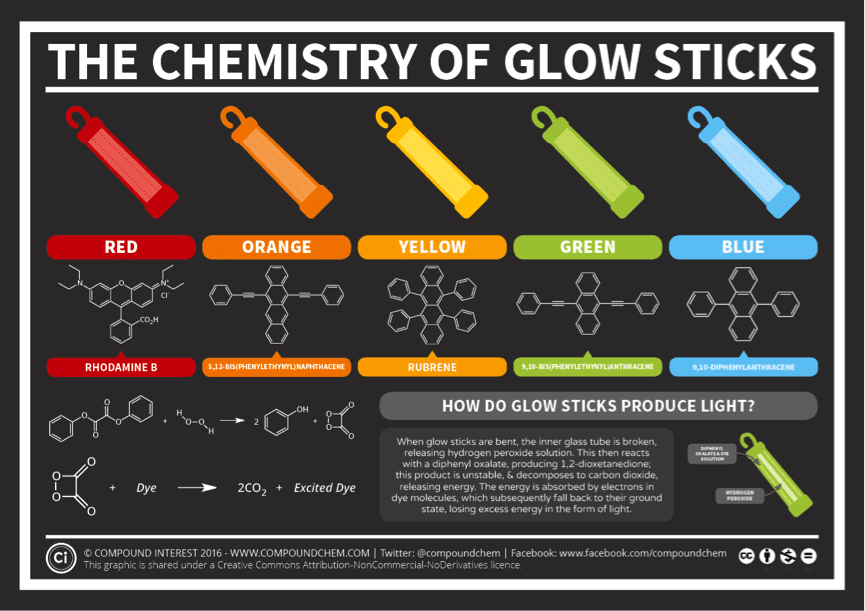
How Are Glow Sticks Made . a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. from formulation to the final glow, we guide you through each step of the. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. Glow sticks actually contain two separate compartments, with two different chemical solutions. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. but how does this process work, and why do you need to bend the glow stick to initiate it? The cyclic peroxy compound decomposes to carbon dioxide. specifically, the chemical reaction works like this: One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. Some absorb energy and some release energy. There are many different kinds of chemical reactions.
from www.glowtopia.co.uk
but how does this process work, and why do you need to bend the glow stick to initiate it? Some absorb energy and some release energy. from formulation to the final glow, we guide you through each step of the. There are many different kinds of chemical reactions. specifically, the chemical reaction works like this: The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. The cyclic peroxy compound decomposes to carbon dioxide. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Glow sticks actually contain two separate compartments, with two different chemical solutions. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing.
How Glow Sticks Work The Science behind glow sticks Glowtopia
How Are Glow Sticks Made Some absorb energy and some release energy. but how does this process work, and why do you need to bend the glow stick to initiate it? from formulation to the final glow, we guide you through each step of the. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. specifically, the chemical reaction works like this: Glow sticks actually contain two separate compartments, with two different chemical solutions. Some absorb energy and some release energy. There are many different kinds of chemical reactions. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. The cyclic peroxy compound decomposes to carbon dioxide.
From www.youtube.com
How to make glowing stick at home DIY glow stick YouTube How Are Glow Sticks Made There are many different kinds of chemical reactions. but how does this process work, and why do you need to bend the glow stick to initiate it? Some absorb energy and some release energy. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic. How Are Glow Sticks Made.
From science.wonderhowto.com
How to Make Your Own Homemade Glow Sticks « Science Experiments How Are Glow Sticks Made There are many different kinds of chemical reactions. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. from formulation to the final glow, we guide you through each step of the. specifically, the chemical reaction works like this: The hydrogen peroxide oxidizes the phenyl oxalate ester, to. How Are Glow Sticks Made.
From www.pinterest.com
Glow Stick Idea for Kids Glow stick ideas, Cool glow, Glow sticks How Are Glow Sticks Made specifically, the chemical reaction works like this: The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. There are many different kinds of chemical reactions. The hydrogen peroxide oxidizes the phenyl. How Are Glow Sticks Made.
From www.youtube.com
How To Multicolored Reusable Glow Sticks or "Glowsticks." Easy project How Are Glow Sticks Made but how does this process work, and why do you need to bend the glow stick to initiate it? There are many different kinds of chemical reactions. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. from formulation to the final glow,. How Are Glow Sticks Made.
From astrocamp.org
How Do Glow Sticks Work? AstroCamp Science Camp How Are Glow Sticks Made Some absorb energy and some release energy. Glow sticks actually contain two separate compartments, with two different chemical solutions. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. One solution, in the case. How Are Glow Sticks Made.
From awesci.com
Making Glow Sticks at Home is Fairly Easy How Are Glow Sticks Made Some absorb energy and some release energy. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. Glow sticks actually contain two separate compartments, with two different chemical solutions. specifically,. How Are Glow Sticks Made.
From www.lihpao.com
When Were Glow Sticks Invented? A Look at the History and Impact of How Are Glow Sticks Made Some absorb energy and some release energy. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. but how does this process work, and why do you need to bend the glow stick to initiate it? what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. specifically, the. How Are Glow Sticks Made.
From glowinthedarkness.com
How to make a glow stick man costume with glow sticks, DIY How Are Glow Sticks Made The cyclic peroxy compound decomposes to carbon dioxide. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. from formulation to the final glow, we guide you through each step of the. specifically, the chemical reaction works like this: Some absorb energy and some release energy. One solution, in the case of most glow sticks,. How Are Glow Sticks Made.
From www.pinterest.com
How to Make Glowing Jars (Using Glow Sticks) Crafty Morning Glow How Are Glow Sticks Made One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. The cyclic peroxy compound decomposes to carbon dioxide. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. what do fireflies, tvs, neon signs,. How Are Glow Sticks Made.
From www.instructables.com
Make Glow Sticks the Science (with Pictures) Instructables How Are Glow Sticks Made specifically, the chemical reaction works like this: The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Glow sticks actually contain two separate compartments, with two different chemical solutions. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. but how does this process work,. How Are Glow Sticks Made.
From science.wonderhowto.com
HowTo DIY Glow Sticks « Science Experiments WonderHowTo How Are Glow Sticks Made The cyclic peroxy compound decomposes to carbon dioxide. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. but how does this process work, and why do you need to bend the glow stick to initiate it? Some absorb energy and some release energy. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and. How Are Glow Sticks Made.
From www.glowtopia.co.uk
How Glow Sticks Work The Science behind glow sticks Glowtopia How Are Glow Sticks Made a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. Glow sticks actually contain two separate compartments, with two different chemical solutions. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. specifically, the chemical reaction works like this: One solution, in the. How Are Glow Sticks Made.
From studydemandants.z21.web.core.windows.net
How Does Glow Stick Work How Are Glow Sticks Made The cyclic peroxy compound decomposes to carbon dioxide. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. but how does this process work, and why do you need to bend the glow stick to initiate it? The hydrogen peroxide oxidizes the phenyl oxalate. How Are Glow Sticks Made.
From www.youtube.com
The Science Of Glow Sticks YouTube How Are Glow Sticks Made from formulation to the final glow, we guide you through each step of the. Some absorb energy and some release energy. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. specifically, the chemical reaction works like this: a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger. How Are Glow Sticks Made.
From www.wikihow.com
How to Make Glow Sticks Glow Again 8 Steps (with Pictures) How Are Glow Sticks Made but how does this process work, and why do you need to bend the glow stick to initiate it? what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. from formulation to the final glow, we guide you through each step of the. Glow sticks actually contain two separate compartments, with two different chemical. How Are Glow Sticks Made.
From science.wonderhowto.com
How to Make Your Own Homemade Glow Sticks « Science Experiments How Are Glow Sticks Made but how does this process work, and why do you need to bend the glow stick to initiate it? The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the. How Are Glow Sticks Made.
From www.glowtopia.co.uk
The Ultimate List Of Glow Stick Ideas Glowtopia How Are Glow Sticks Made what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. Some absorb energy and some release energy. The cyclic peroxy compound decomposes to carbon dioxide. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. from formulation to the final glow, we guide you through each step of the.. How Are Glow Sticks Made.
From owlcation.com
How Do Glow Sticks Work? Owlcation How Are Glow Sticks Made The cyclic peroxy compound decomposes to carbon dioxide. Glow sticks actually contain two separate compartments, with two different chemical solutions. specifically, the chemical reaction works like this: The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. a glow stick consists of a glass vial, containing one chemical solution, housed inside a. How Are Glow Sticks Made.
From www.pinterest.com
Make Reusable Glow Stick Cubes Glowing Colors DIY Glow sticks, Glow How Are Glow Sticks Made Some absorb energy and some release energy. Glow sticks actually contain two separate compartments, with two different chemical solutions. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. specifically, the chemical reaction works like this: One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose. How Are Glow Sticks Made.
From science.wonderhowto.com
How to Make Your Own Homemade Glow Sticks « Science Experiments How Are Glow Sticks Made Glow sticks actually contain two separate compartments, with two different chemical solutions. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. specifically, the chemical reaction works like this: from formulation to the final glow, we guide you through each step of the.. How Are Glow Sticks Made.
From www.youtube.com
Making glow sticks from scratch YouTube How Are Glow Sticks Made The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Some absorb energy and some release energy. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the. How Are Glow Sticks Made.
From www.lihpao.com
How Does a Glow Stick Work? Exploring the Chemistry and Physics Behind How Are Glow Sticks Made One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. specifically, the chemical reaction works like this: There are many different kinds of chemical. How Are Glow Sticks Made.
From www.instructables.com
"Glow Stick" 9 Steps Instructables How Are Glow Sticks Made The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Glow sticks actually contain two separate compartments, with two different chemical solutions. The cyclic peroxy compound decomposes to carbon dioxide. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. There are many different kinds of chemical reactions. The hydrogen peroxide oxidizes the. How Are Glow Sticks Made.
From glowdecoration.com
How to make your own glow sticks Glow Decorations How Are Glow Sticks Made a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. but how does this process work, and why do you need to bend the glow stick to initiate it? what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. Some absorb energy and some release. How Are Glow Sticks Made.
From www.pinterest.co.uk
How Do Glow Sticks Work? How Are Glow Sticks Made a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. from formulation to the final glow, we guide you through each step of the. There are many different kinds of chemical reactions. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. Glow sticks actually. How Are Glow Sticks Made.
From www.wikihow.com
How to Make Glow Sticks Glow Again 8 Steps (with Pictures) How Are Glow Sticks Made but how does this process work, and why do you need to bend the glow stick to initiate it? Glow sticks actually contain two separate compartments, with two different chemical solutions. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. One solution, in the case of most glow sticks, contains a diphenyl. How Are Glow Sticks Made.
From www.etsy.com
Neon Light Glow Sticks Party Pack Glow Sticks With Connector Etsy How Are Glow Sticks Made what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. from formulation to the final glow, we guide you through each step of the. Glow sticks actually contain two separate compartments, with two different chemical solutions. The cyclic peroxy compound decomposes to carbon dioxide. but how does this process work, and why do you. How Are Glow Sticks Made.
From www.youtube.com
Make Reusable Glow Sticks Safe Easy & No Chemicals Long Lasting How Are Glow Sticks Made but how does this process work, and why do you need to bend the glow stick to initiate it? Some absorb energy and some release energy. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an. How Are Glow Sticks Made.
From www.youtube.com
Homemade Glow Stick YouTube How Are Glow Sticks Made There are many different kinds of chemical reactions. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. The cyclic peroxy compound decomposes to carbon dioxide. Glow sticks actually contain two separate compartments, with two different chemical solutions. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye. How Are Glow Sticks Made.
From www.youtube.com
Make Reusable Glow Sticks YouTube How Are Glow Sticks Made Glow sticks actually contain two separate compartments, with two different chemical solutions. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. but how does this process work, and why do you need to bend the glow stick to initiate it? specifically, the chemical reaction works like this:. How Are Glow Sticks Made.
From www.pinterest.com
Fun Glow Stick Ideas in 2021 Cheap diy crafts, Kid friendly diy, Glow How Are Glow Sticks Made The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. a glow stick consists. How Are Glow Sticks Made.
From www.thoughtco.com
How Glow Stick Colors Work How Are Glow Sticks Made but how does this process work, and why do you need to bend the glow stick to initiate it? what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. from formulation to the final glow, we guide you through each step of the. There are many different kinds of chemical reactions. Glow sticks actually. How Are Glow Sticks Made.
From www.thoughtco.com
How Glow Stick Colors Work How Are Glow Sticks Made Some absorb energy and some release energy. specifically, the chemical reaction works like this: The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. but how does this process work, and why do you need to bend the glow stick to initiate it? Glow sticks actually contain two separate compartments, with two different chemical solutions.. How Are Glow Sticks Made.
From www.youtube.com
GlowFun Glow Sticks How to activate glow sticks YouTube How Are Glow Sticks Made One solution, in the case of most glow sticks, contains a diphenyl oxalate compound, along with a dye whose identity varies depending on the desired. There are many different kinds of chemical reactions. specifically, the chemical reaction works like this: The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. Glow sticks actually. How Are Glow Sticks Made.
From www.wikihow.com
How to Make Glow in the Dark Sticks Last Longer 4 Steps How Are Glow Sticks Made what do fireflies, tvs, neon signs, and glowsticks have in common?learn more at. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. a glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing. Some absorb energy and some release energy. from formulation to the final. How Are Glow Sticks Made.