Bromine Water Is Reaction . Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Alkanes and alkenes have different molecular structures; Bromine water is an orange solution of bromine. All alkanes are saturated and alkenes are unsaturated; Alkenes can decolourise bromine water,. [1] it is often used as. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. The presence of the c=c double bond allows. In essence, the electrophilic pi bond in the. These reactions are usually spontaneous. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. It becomes colourless when it is shaken with an alkene. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). Chlorine, bromine or iodine can be added to an alkene.
from www.numerade.com
[1] it is often used as. The presence of the c=c double bond allows. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. It becomes colourless when it is shaken with an alkene. Alkenes can decolourise bromine water,. Alkanes and alkenes have different molecular structures; Chlorine, bromine or iodine can be added to an alkene. In essence, the electrophilic pi bond in the. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o).
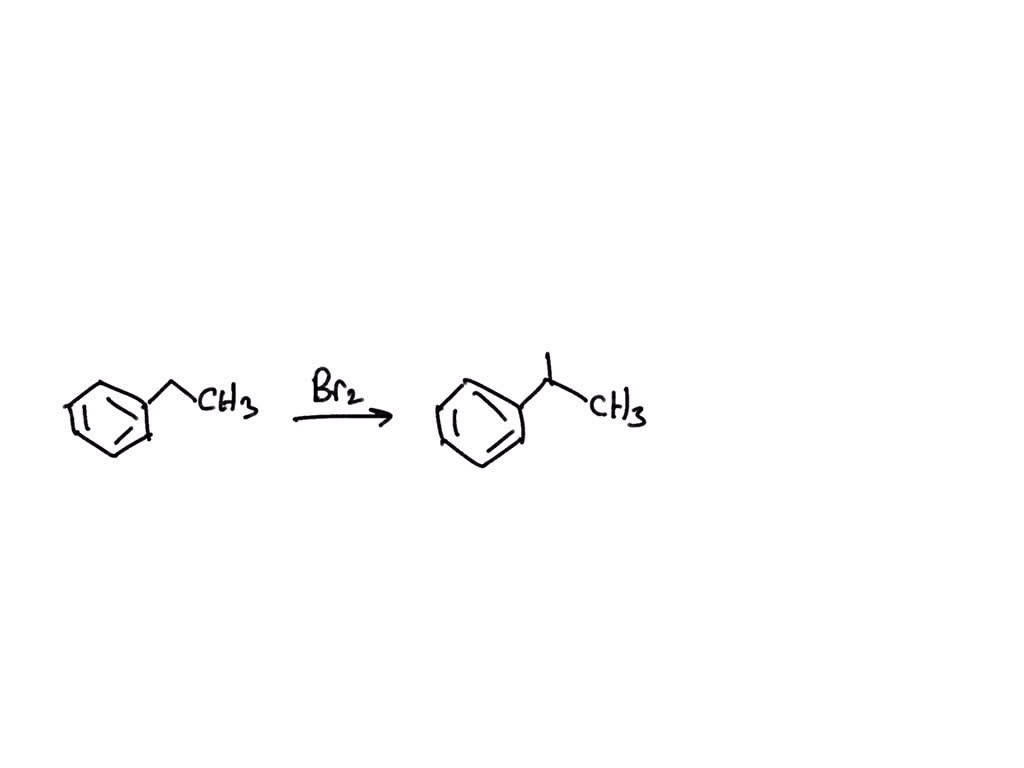
SOLVED What is the major product of the reaction between ethylbenzene
Bromine Water Is Reaction In essence, the electrophilic pi bond in the. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). [1] it is often used as. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkanes and alkenes have different molecular structures; It becomes colourless when it is shaken with an alkene. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. The presence of the c=c double bond allows. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. These reactions are usually spontaneous. Chlorine, bromine or iodine can be added to an alkene. All alkanes are saturated and alkenes are unsaturated; Alkenes can decolourise bromine water,. Bromine water is an orange solution of bromine. In essence, the electrophilic pi bond in the.
From hoachathaidang.com
BÁN BROMINE WATER (CONCENTRATED), ALPHA CHEMIKA HÓA CHẤT HẢI ĐĂNG Bromine Water Is Reaction Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Alkanes and alkenes have different molecular structures; When bromine is added to the sample, if the reddish color disappear, that. Bromine Water Is Reaction.
From www.sciencephoto.com
Test tube of bromine water Stock Image C029/0952 Science Photo Bromine Water Is Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. These reactions are usually spontaneous. Alkenes can decolourise bromine water,. [1] it is often used as. It becomes colourless when it is shaken with an alkene. The presence of the c=c double bond allows. The products for addition of. Bromine Water Is Reaction.
From www.nagwa.com
Question Video Describing the Reaction of Phenol and Alkenes with Bromine Water Is Reaction Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). The presence of the c=c double bond allows. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. It becomes colourless when it is shaken with an alkene. Chlorine, bromine. Bromine Water Is Reaction.
From ar.inspiredpencil.com
Bromine Water Bromine Water Is Reaction All alkanes are saturated and alkenes are unsaturated; Bromine water is an orange solution of bromine. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. These reactions are. Bromine Water Is Reaction.
From dokumen.tips
(PDF) Bromine Water, Saturated LabChem Inc · Bromine Water, Saturated Bromine Water Is Reaction The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. In essence, the electrophilic pi bond in the. Bromine water is an orange solution of bromine. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). Alkanes and alkenes have different molecular structures; When. Bromine Water Is Reaction.
From www.vedantu.com
Reaction of bromine water phenol gives white ppt ofA. o bromophenolB Bromine Water Is Reaction Chlorine, bromine or iodine can be added to an alkene. All alkanes are saturated and alkenes are unsaturated; The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Bromine water is an orange solution of bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Water Is Reaction.
From www.flickriver.com
Bromine water a photo on Flickriver Bromine Water Is Reaction Chlorine, bromine or iodine can be added to an alkene. Bromine water is an orange solution of bromine. The presence of the c=c double bond allows. It becomes colourless when it is shaken with an alkene. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Alkanes and alkenes have different. Bromine Water Is Reaction.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 4.4.2 Bromine & Alkenes翰林国际教育 Bromine Water Is Reaction The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. In essence, the electrophilic pi bond in the. When bromine is added to the sample, if the reddish color disappear,. Bromine Water Is Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Water Is Reaction Chlorine, bromine or iodine can be added to an alkene. It becomes colourless when it is shaken with an alkene. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. These reactions are usually spontaneous. Bromine water is an orange solution of bromine. Bromine water is an oxidizing, intense brown mixture. Bromine Water Is Reaction.
From ar.inspiredpencil.com
Bromine Water Bromine Water Is Reaction [1] it is often used as. The presence of the c=c double bond allows. Alkanes and alkenes have different molecular structures; All alkanes are saturated and alkenes are unsaturated; The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Alkenes can decolourise bromine water,. These reactions are usually spontaneous. Bromine water is an. Bromine Water Is Reaction.
From kortynh.blogspot.com
Bromine Water Test Equation / Bromine Water Test Tryptophan Alcohol Bromine Water Is Reaction Bromine water is an orange solution of bromine. Chlorine, bromine or iodine can be added to an alkene. [1] it is often used as. Alkanes and alkenes have different molecular structures; Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Alkenes can decolourise bromine water,. When bromine is. Bromine Water Is Reaction.
From www.coursehero.com
[Solved] The reaction between bromine and ethane occurs in the presence Bromine Water Is Reaction The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. All alkanes are saturated and alkenes are unsaturated; When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Water Is Reaction.
From www.numerade.com
SOLVED What is the major product of the reaction between ethylbenzene Bromine Water Is Reaction Chlorine, bromine or iodine can be added to an alkene. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. The presence of the c=c double bond allows. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkanes and alkenes. Bromine Water Is Reaction.
From dir.indiamart.com
Bromine Water at Best Price in India Bromine Water Is Reaction Bromine water is an orange solution of bromine. Alkanes and alkenes have different molecular structures; The presence of the c=c double bond allows. [1] it is often used as. All alkanes are saturated and alkenes are unsaturated; Chlorine, bromine or iodine can be added to an alkene. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2). Bromine Water Is Reaction.
From www.nagwa.com
Question Video Identifying the Product of the Reaction of Ethyne Gas Bromine Water Is Reaction Bromine water is an orange solution of bromine. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. All alkanes are saturated and alkenes are unsaturated; [1] it is often used as. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o).. Bromine Water Is Reaction.
From www.chegg.com
Solved When ethene is bubbled through bromine water, the Bromine Water Is Reaction In essence, the electrophilic pi bond in the. The presence of the c=c double bond allows. All alkanes are saturated and alkenes are unsaturated; Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off.. Bromine Water Is Reaction.
From www.sliderbase.com
CnH2n Alkenes Cyclo alkanes Unsaturated Saturated C4H8 C4H8 Слайд Bromine Water Is Reaction When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. In essence, the electrophilic pi bond in the. Bromine water is an orange solution of bromine. The products for addition of halogen to. Bromine Water Is Reaction.
From www.coursehero.com
[Solved] what happen if you combine 1 ml bromine water and few drops Bromine Water Is Reaction It becomes colourless when it is shaken with an alkene. The presence of the c=c double bond allows. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. [1] it is often used as. In essence, the electrophilic pi bond in the. Bromine water is an orange solution of bromine. The products for. Bromine Water Is Reaction.
From chiangmaiplaces.net
Is Bromine Water An Oxidising Agent? Trust The Answer Bromine Water Is Reaction Chlorine, bromine or iodine can be added to an alkene. It becomes colourless when it is shaken with an alkene. These reactions are usually spontaneous. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. [1] it is often used as. Alkanes and alkenes have different molecular structures; Bromine water. Bromine Water Is Reaction.
From dawson-bogspotsheppard.blogspot.com
Bromine Water Test Equation Bromine Water Is Reaction The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. [1] it is often used as. These reactions are usually spontaneous. All alkanes are saturated and alkenes are unsaturated; Alkenes can decolourise bromine water,. Bromine water is an orange solution of bromine. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine. Bromine Water Is Reaction.
From www.livescience.com
Facts About Bromine Live Science Bromine Water Is Reaction Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). It becomes colourless when it is shaken with an alkene. These reactions are usually spontaneous. The presence of the c=c double bond allows. [1] it is often used as. Alkenes react in the cold with pure liquid bromine, or with a. Bromine Water Is Reaction.
From edu.rsc.org
Handling liquid bromine and preparing bromine water Experiment RSC Bromine Water Is Reaction The presence of the c=c double bond allows. In essence, the electrophilic pi bond in the. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Alkanes and alkenes have different molecular structures; Chlorine, bromine or iodine can be added to an alkene. When bromine is added to the sample, if the reddish. Bromine Water Is Reaction.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Water Is Reaction Alkanes and alkenes have different molecular structures; In essence, the electrophilic pi bond in the. Alkenes can decolourise bromine water,. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). [1] it. Bromine Water Is Reaction.
From ar.inspiredpencil.com
Bromine Water Bromine Water Is Reaction The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Chlorine, bromine or iodine can be added to an alkene. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes can decolourise bromine water,. Alkanes and alkenes have different molecular. Bromine Water Is Reaction.
From www.numerade.com
SOLVED When butene is treated with liquid bromine under aqueous Bromine Water Is Reaction The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. It becomes colourless when it is shaken with an alkene. Alkanes and alkenes have different molecular structures; These reactions are usually spontaneous.. Bromine Water Is Reaction.
From chemistry.stackexchange.com
organic chemistry Halogenation of Phenol Chemistry Stack Exchange Bromine Water Is Reaction These reactions are usually spontaneous. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. It becomes colourless when it is shaken with an alkene. Chlorine, bromine or iodine can be added to an alkene. Bromine water is an orange solution of bromine. All alkanes are saturated and alkenes. Bromine Water Is Reaction.
From www.pinterest.jp
Pin on chem Bromine Water Is Reaction It becomes colourless when it is shaken with an alkene. These reactions are usually spontaneous. Chlorine, bromine or iodine can be added to an alkene. Alkanes and alkenes have different molecular structures; The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. In essence, the electrophilic pi bond in the. The. Bromine Water Is Reaction.
From www.indiamart.com
Bromine Water, Laboratory, 500ml Bottle at Rs 413 in Hyderabad ID Bromine Water Is Reaction The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine water. Bromine Water Is Reaction.
From www.science-revision.co.uk
Electrophilic addition Bromine Water Is Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. These reactions are usually spontaneous. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. [1] it is often used as. Bromine water is an orange solution of bromine. In essence, the. Bromine Water Is Reaction.
From www.linstitute.net
CIE A Level Chemistry复习笔记3.2.9 Test for Unsaturation翰林国际教育 Bromine Water Is Reaction Bromine water is an orange solution of bromine. In essence, the electrophilic pi bond in the. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. These reactions are usually spontaneous. Chlorine, bromine or iodine can be added to an alkene. The presence of the c=c double bond allows.. Bromine Water Is Reaction.
From www.gkch.eu
Bromine GKCH GmbH Bromine Water Is Reaction In essence, the electrophilic pi bond in the. Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. These reactions are usually spontaneous. Chlorine, bromine or iodine can be added to an alkene. Bromine. Bromine Water Is Reaction.
From scienceready.com.au
Bromine Water Test Science Ready Bromine Water Is Reaction Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). Alkanes and alkenes have different molecular structures; The presence of the c=c double bond allows. [1] it is often used as. Alkenes can decolourise bromine water,. In essence, the electrophilic pi bond in the. It becomes colourless when it is shaken. Bromine Water Is Reaction.
From www.youtube.com
Propane reacts with bromine YouTube Bromine Water Is Reaction All alkanes are saturated and alkenes are unsaturated; The presence of the c=c double bond allows. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. In essence, the electrophilic pi bond in the. Chlorine, bromine or iodine can be added to an alkene. It becomes colourless when it. Bromine Water Is Reaction.
From www.labster.com
5 Ways to Teach Bromine Test For Unsaturated Bonds to Students Bromine Water Is Reaction It becomes colourless when it is shaken with an alkene. Alkenes can decolourise bromine water,. In essence, the electrophilic pi bond in the. Bromine water is an orange solution of bromine. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The addition reaction occurs to get reddish bromine consumed. Bromine Water Is Reaction.
From saniyahghopdalton.blogspot.com
Reaction of Cyclohexene With Bromine Bromine Water Is Reaction Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (br 2) dissolved in water (h 2 o). Alkanes and alkenes have different molecular structures; Chlorine, bromine or iodine can be added to an alkene. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The products for addition. Bromine Water Is Reaction.