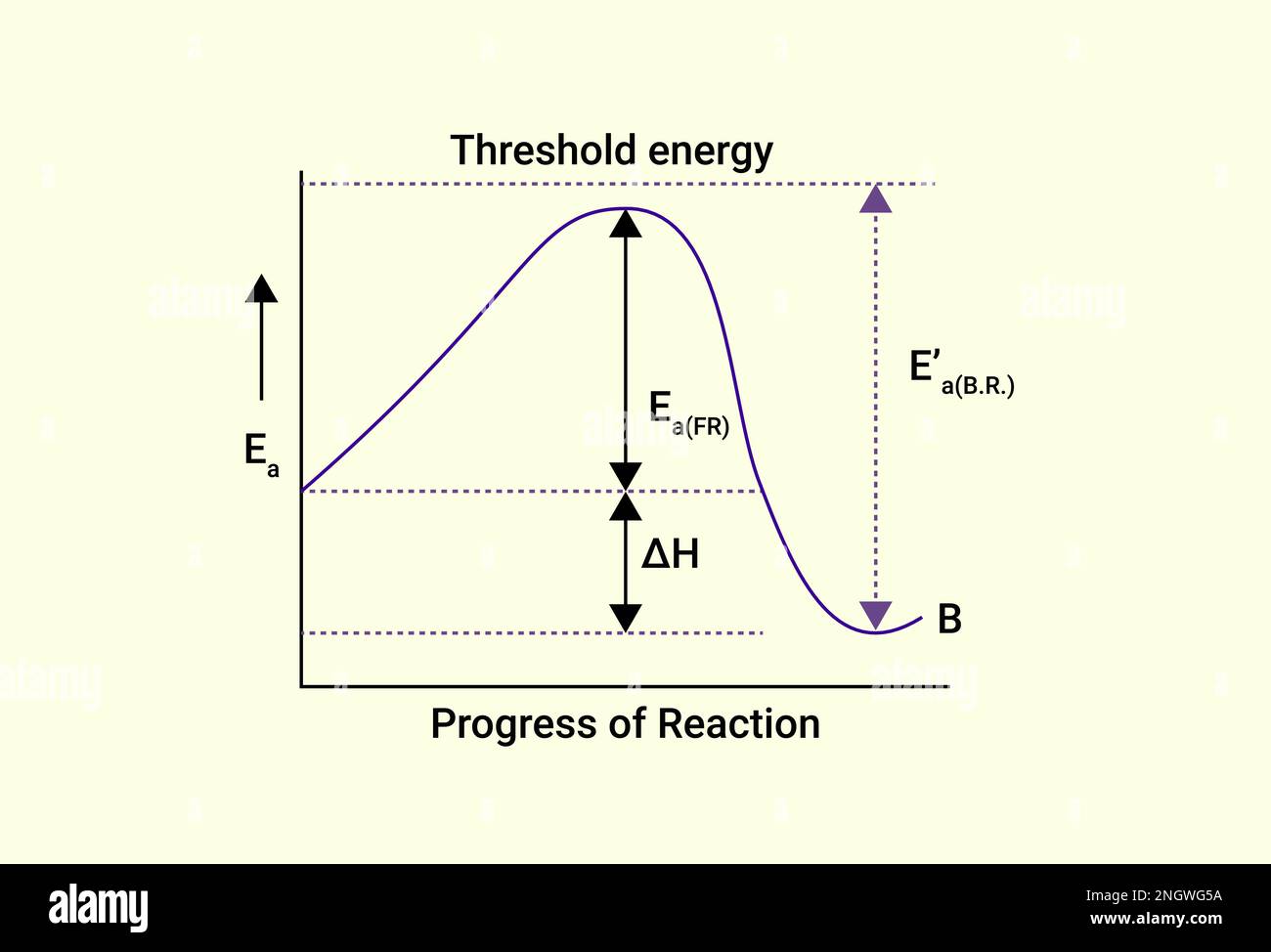
Threshold Energy Graph . The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. This energy threshold, called the. For a chemical reaction to occur, an energy threshold must be. The minimum energy required to release a photoelectron from the surface of a metal. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Consider the electrons in a metal as. The work function φ, or threshold energy, of a material, is defined as: To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The difference between these two values is the activation energy,. The threshold energy is the energy that these molecules must have in order for a reaction to take place.
from www.alamy.com
The work function φ, or threshold energy, of a material, is defined as: The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. The minimum energy required to release a photoelectron from the surface of a metal. The difference between these two values is the activation energy,. Consider the electrons in a metal as. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. For a chemical reaction to occur, an energy threshold must be. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur.
Graph of Progress of Reaction and Threshold energy Stock Vector Image
Threshold Energy Graph The work function φ, or threshold energy, of a material, is defined as: Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The minimum energy required to release a photoelectron from the surface of a metal. For a chemical reaction to occur, an energy threshold must be. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. This energy threshold, called the. The threshold energy is the energy that these molecules must have in order for a reaction to take place. The work function φ, or threshold energy, of a material, is defined as: The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. The difference between these two values is the activation energy,. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Consider the electrons in a metal as.
From www.researchgate.net
The threshold energy E 0 is determined by the equation (3.1). The red Threshold Energy Graph The minimum energy required to release a photoelectron from the surface of a metal. The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Collision theory provides a qualitative. Threshold Energy Graph.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy Graph In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Consider the electrons in a metal as. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy represents the minimum energy required for a chemical reaction to occur,. Threshold Energy Graph.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy Graph The threshold energy is the energy that these molecules must have in order for a reaction to take place. For a chemical reaction to occur, an energy threshold must be. The difference between these two values is the activation energy,. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum. Threshold Energy Graph.
From www.researchgate.net
The laser threshold energy (red) and the slope efficiency (green) as Threshold Energy Graph The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. This energy threshold, called the. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The work function φ, or threshold energy, of a material, is defined. Threshold Energy Graph.
From www.toppr.com
The energy profile for the reaction CO(g) + NO2(g) CO2(g) + NO(g) is Threshold Energy Graph The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. This energy threshold, called the. The work function φ, or threshold energy, of a material, is defined as: To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as. Threshold Energy Graph.
From www.researchgate.net
(a) As in figure 4(a) but far from the threshold energy region (ω/I Threshold Energy Graph For a chemical reaction to occur, an energy threshold must be. This energy threshold, called the. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Consider the electrons in a metal as. To find the threshold is a naive way, we could calculate the velocity of the center. Threshold Energy Graph.
From schematicdataweals77.z13.web.core.windows.net
Energy Diagram Of Exothermic Reaction Threshold Energy Graph The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. This energy threshold, called the. To find the threshold is a naive way, we could calculate the. Threshold Energy Graph.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Threshold Energy Graph The threshold energy is the energy that these molecules must have in order for a reaction to take place. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between. Threshold Energy Graph.
From diagramdatacheyennes.z21.web.core.windows.net
Simple Energy Diagram Threshold Energy Graph This energy threshold, called the. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Consider the electrons in a metal as. The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. Collision theory provides a qualitative. Threshold Energy Graph.
From fixportillobadgered.z21.web.core.windows.net
Energy Diagram Activation Energy Threshold Energy Graph Consider the electrons in a metal as. The minimum energy required to release a photoelectron from the surface of a metal. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. To find the threshold is a naive way, we could calculate the velocity of the center of momentum. Threshold Energy Graph.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate Threshold Energy Graph The threshold energy is the energy that these molecules must have in order for a reaction to take place. Consider the electrons in a metal as. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The minimum energy required to release a photoelectron from the surface of a metal. This energy threshold, called. Threshold Energy Graph.
From manualdataesurient.z21.web.core.windows.net
Energy Level Diagram For Exothermic Reaction Threshold Energy Graph Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. For a chemical reaction to occur, an energy threshold must be. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy represents the minimum energy required for a chemical reaction to occur, while. Threshold Energy Graph.
From www.researchgate.net
Variations of remaining energy and threshold energy against prediction Threshold Energy Graph For a chemical reaction to occur, an energy threshold must be. The work function φ, or threshold energy, of a material, is defined as: The threshold energy is the energy that these molecules must have in order for a reaction to take place. This energy threshold, called the. Consider the electrons in a metal as. To find the threshold is. Threshold Energy Graph.
From www.vedantu.com
Photoelectric Effect and Stopping Potential Important Concepts and Tips Threshold Energy Graph Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. For a chemical reaction to occur, an energy threshold must be. The difference between these two values is the activation energy,. The minimum energy required to release a photoelectron from the surface of a metal. The work function φ, or threshold energy, of a. Threshold Energy Graph.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy Graph The difference between these two values is the activation energy,. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Activation energy represents the minimum energy required for a chemical reaction to occur,. Threshold Energy Graph.
From issuu.com
Transition State Calculation by CD ComputaBio Issuu Threshold Energy Graph Consider the electrons in a metal as. For a chemical reaction to occur, an energy threshold must be. This energy threshold, called the. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have. Threshold Energy Graph.
From www.researchgate.net
The threshold energy, √ s th , for K + Λ and K + Σ Download Threshold Energy Graph The threshold energy is the energy that these molecules must have in order for a reaction to take place. This energy threshold, called the. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The minimum energy required to release a photoelectron from the surface of a metal. The. Threshold Energy Graph.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Threshold Energy Graph For a chemical reaction to occur, an energy threshold must be. This energy threshold, called the. The work function φ, or threshold energy, of a material, is defined as: To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The activation energy (\(e_a\)),. Threshold Energy Graph.
From www.researchgate.net
Threshold energy density for transition in Poiseuille ow for the three Threshold Energy Graph This energy threshold, called the. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The difference between these two values is the activation energy,. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Collision theory provides a qualitative. Threshold Energy Graph.
From www.researchgate.net
The 2s 2 1 S e resonance energies E r of a He atom and the threshold Threshold Energy Graph In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The work function φ, or threshold energy, of a material, is defined as: The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. The minimum energy required. Threshold Energy Graph.
From www.researchgate.net
Comparison of specific threshold energy (J/kg). Download Scientific Threshold Energy Graph For a chemical reaction to occur, an energy threshold must be. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The work function φ, or threshold energy, of a material, is defined as: The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the. Threshold Energy Graph.
From courses.lumenlearning.com
The Photoelectric Effect Physics Threshold Energy Graph To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. Activation energy represents the minimum energy required for a chemical reaction to. Threshold Energy Graph.
From www.researchgate.net
Burst duration versus the threshold energy of gammaray photons for Threshold Energy Graph This energy threshold, called the. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The work function φ, or threshold energy, of a material, is defined as: Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy.. Threshold Energy Graph.
From www.researchgate.net
Threshold energy E th of the reticulation plotted versus the distance z Threshold Energy Graph The threshold energy is the energy that these molecules must have in order for a reaction to take place. In chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The minimum energy required to release a photoelectron from the surface of a metal. The difference between these two values. Threshold Energy Graph.
From www.researchgate.net
The predicted integrated flux above the threshold energy E γ , with an Threshold Energy Graph Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy is the energy that these molecules must have in order for a reaction to take place. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as. Threshold Energy Graph.
From www.topperlearning.com
20 the data given below gives the photon energy in ev for a number of Threshold Energy Graph The difference between these two values is the activation energy,. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Consider the electrons in a metal as. For a chemical reaction to occur, an energy threshold must be. To find the threshold is a naive way, we could calculate the velocity of the center. Threshold Energy Graph.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Threshold Energy Graph To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The minimum energy required to release a photoelectron from the surface of. Threshold Energy Graph.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Threshold Energy Graph Consider the electrons in a metal as. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The work function φ, or. Threshold Energy Graph.
From www.researchgate.net
(Color online). Schematic of the threshold energy for pairbreaking Threshold Energy Graph This energy threshold, called the. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Consider the electrons in a metal as. In chemical reactions, the energy barrier corresponds to the amount of energy the. Threshold Energy Graph.
From www.researchgate.net
Threshold energy n of the lowest three subbands as a function of 2. The Threshold Energy Graph To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The work function φ, or threshold energy, of a material, is defined as: The threshold energy is. Threshold Energy Graph.
From lessonschooluninserted.z14.web.core.windows.net
How To Read An Energy Diagram Threshold Energy Graph The difference between these two values is the activation energy,. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The threshold energy is the energy that these molecules must have in order for a reaction to take place. The activation energy (\(e_a\)),. Threshold Energy Graph.
From mungfali.com
Exothermic Reaction Energy Profile Diagram Threshold Energy Graph For a chemical reaction to occur, an energy threshold must be. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The work function φ, or threshold energy, of a material, is defined as: To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as. Threshold Energy Graph.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy Graph To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The minimum energy required to release a photoelectron from the surface of a metal. The threshold energy is the energy that these molecules must have in order for a reaction to take place.. Threshold Energy Graph.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Energy Graph Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known. This energy threshold, called the. To find the threshold is a naive way, we could calculate the. Threshold Energy Graph.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy Graph The threshold energy is the energy that these molecules must have in order for a reaction to take place. For a chemical reaction to occur, an energy threshold must be. This energy threshold, called the. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. To find the threshold is a naive way, we. Threshold Energy Graph.