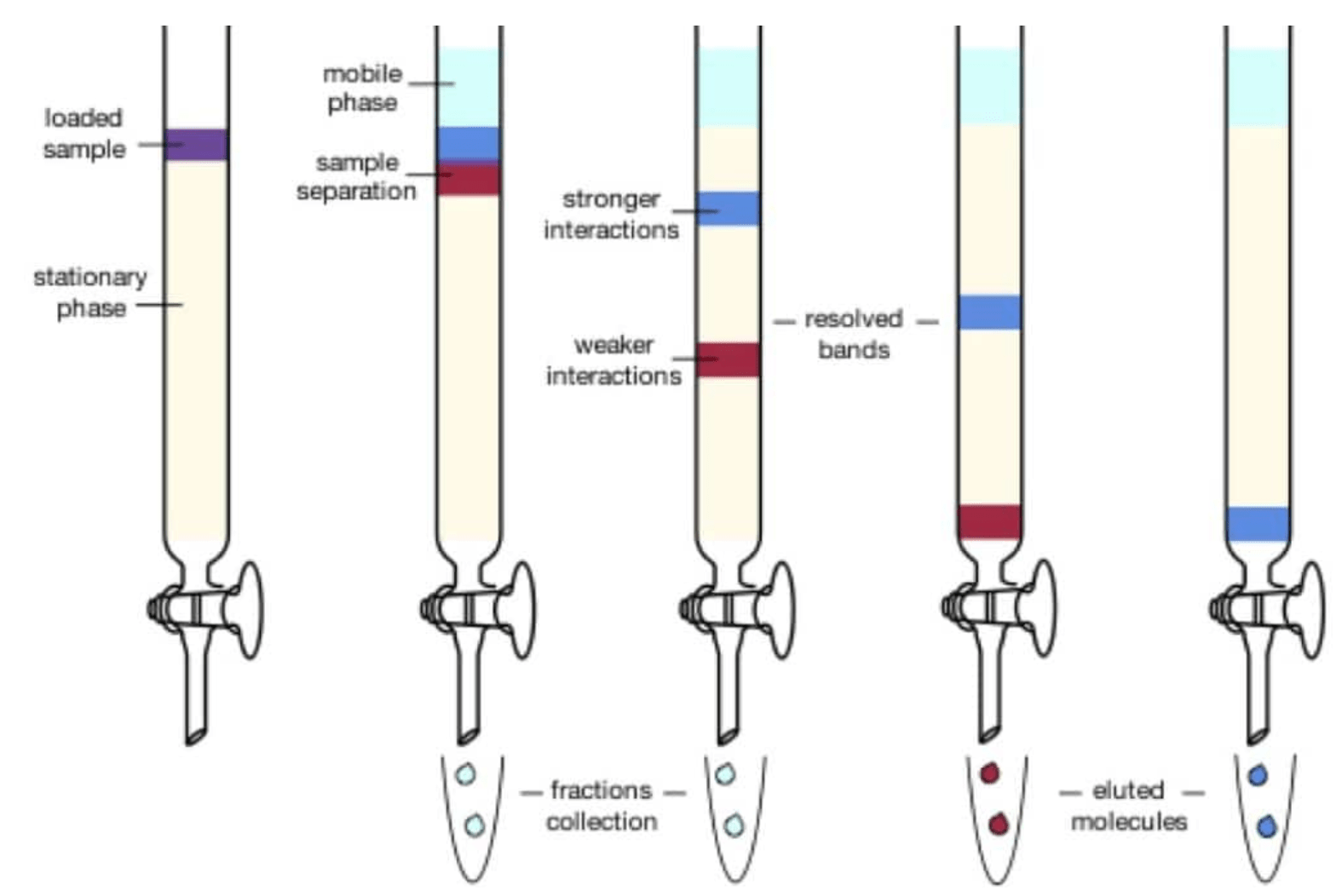
Chromatography Examples . chromatography is a method by which a mixture is separated by distributing its components between two phases. four examples of interactions between a solute and the stationary phase: For each example, the smaller, green solute is more strongly retained than the larger, red solute. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. The stationary phase remains fixed in place while the mobile phase carries the components. learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Learn more about chromatography in this article.
from
learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. For each example, the smaller, green solute is more strongly retained than the larger, red solute. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography is a method by which a mixture is separated by distributing its components between two phases. Learn more about chromatography in this article. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. four examples of interactions between a solute and the stationary phase:
Chromatography Examples For each example, the smaller, green solute is more strongly retained than the larger, red solute. learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography is a method by which a mixture is separated by distributing its components between two phases. four examples of interactions between a solute and the stationary phase: For each example, the smaller, green solute is more strongly retained than the larger, red solute. Learn more about chromatography in this article. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture.
From www.slideserve.com
PPT Applications in Forensic Science PowerPoint Presentation ID225831 Chromatography Examples Learn more about chromatography in this article. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. The stationary phase remains fixed in place while the mobile phase carries the components.. Chromatography Examples.
From
Chromatography Examples learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each. Chromatography Examples.
From paksc.org
Simplest Chromatography Experiment Do Science! Chromatography Examples chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. four examples of interactions between a solute and the stationary phase: Learn more about chromatography in this article. learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. For each. Chromatography Examples.
From
Chromatography Examples The stationary phase remains fixed in place while the mobile phase carries the components. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. chromatography is a method by which a mixture is separated by distributing its components between two phases. learn about the basic principle, types, and. Chromatography Examples.
From
Chromatography Examples chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving. Chromatography Examples.
From
Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Learn more about chromatography in this article. For each example, the smaller, green solute is more strongly retained than the larger, red solute.. Chromatography Examples.
From cartoondealer.com
Paper Chromatography Analytical Method For The Separation Of A Mixture Chromatography Examples The stationary phase remains fixed in place while the mobile phase carries the components. learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid. Chromatography Examples.
From
Chromatography Examples learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. For each example, the smaller, green solute is more strongly retained than the larger, red solute. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. chromatography, technique for separating. Chromatography Examples.
From en.wikipedia.org
Thinlayer chromatography Wikipedia Chromatography Examples learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. chromatography is a. Chromatography Examples.
From
Chromatography Examples chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile phase carries the components. Learn more about chromatography in this article. four examples of interactions between a solute and the stationary phase: For each example, the smaller, green solute is more. Chromatography Examples.
From blog.edvotek.com
Thin Layer Chromatography The Official Blog of Edvotek® Chromatography Examples learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. Learn more about chromatography in this article. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a. Chromatography Examples.
From www.slideserve.com
PPT CHROMATOGRAPHY PowerPoint Presentation, free download ID6719683 Chromatography Examples four examples of interactions between a solute and the stationary phase: The stationary phase remains fixed in place while the mobile phase carries the components. Learn more about chromatography in this article. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid. Chromatography Examples.
From
Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. learn about. Chromatography Examples.
From
Chromatography Examples four examples of interactions between a solute and the stationary phase: chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography, technique for separating the components, or solutes, of a mixture on the basis of. Chromatography Examples.
From bio.libretexts.org
2.4 Chromatography Biology LibreTexts Chromatography Examples chromatography is a method by which a mixture is separated by distributing its components between two phases. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. four examples. Chromatography Examples.
From
Chromatography Examples The stationary phase remains fixed in place while the mobile phase carries the components. For each example, the smaller, green solute is more strongly retained than the larger, red solute. Learn more about chromatography in this article. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed. Chromatography Examples.
From
Chromatography Examples chromatography is a method by which a mixture is separated by distributing its components between two phases. Learn more about chromatography in this article. learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography. Chromatography Examples.
From www.priyamstudycentre.com
Chromatography Techniques, Definition, Principle, Types Chromatography Examples chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. For each example, the smaller, green solute is more strongly retained than the larger, red solute. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography, technique for separating the components, or solutes, of. Chromatography Examples.
From brittanywashburn.com
Chromatography Separating a Solution Chromatography Examples learn about the basic principle, types, and applications of chromatography, a biophysical technique for separating and analyzing mixtures. chromatography is a method by which a mixture is separated by distributing its components between two phases. For each example, the smaller, green solute is more strongly retained than the larger, red solute. Learn more about chromatography in this article.. Chromatography Examples.
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography is a group of laboratory techniques used. Chromatography Examples.
From
Chromatography Examples The stationary phase remains fixed in place while the mobile phase carries the components. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. chromatography is a method by which a mixture is separated by distributing its components between two phases. Learn more about chromatography in this article. . Chromatography Examples.
From
Chromatography Examples The stationary phase remains fixed in place while the mobile phase carries the components. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. chromatography is a group of laboratory techniques used. Chromatography Examples.
From
Chromatography Examples The stationary phase remains fixed in place while the mobile phase carries the components. four examples of interactions between a solute and the stationary phase: chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. Learn more about chromatography in this article. learn about the basic principle, types,. Chromatography Examples.
From www.slideserve.com
PPT Column Chromatography PowerPoint Presentation, free download ID Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. four examples. Chromatography Examples.
From www.kirikcitarim.com
纸张色谱仪表微生物纸币 Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Learn more about chromatography in this article. For each example, the smaller, green solute is more strongly retained than the larger, red solute.. Chromatography Examples.
From
Chromatography Examples Learn more about chromatography in this article. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts. Chromatography Examples.
From www.slideshare.net
Chromatography Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. The stationary phase remains fixed in place while the mobile phase carries the components. Learn more about chromatography in this article. chromatography. Chromatography Examples.
From www.birmingham.ac.uk
Biology electrophoresis University of Birmingham Chromatography Examples four examples of interactions between a solute and the stationary phase: For each example, the smaller, green solute is more strongly retained than the larger, red solute. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. The stationary phase remains fixed in place while the mobile phase carries. Chromatography Examples.
From
Chromatography Examples chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. learn about. Chromatography Examples.
From
Chromatography Examples chromatography is a method by which a mixture is separated by distributing its components between two phases. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. four examples of interactions between a solute and the stationary phase: The stationary phase remains fixed in place while the mobile. Chromatography Examples.
From
Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. chromatography is a method by which a mixture is separated by distributing its components between two phases. learn about the basic. Chromatography Examples.
From www.wellappointeddesk.com
Ink Chromatography The WellAppointed Desk Chromatography Examples four examples of interactions between a solute and the stationary phase: chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. learn about the basic principle, types, and applications of chromatography,. Chromatography Examples.
From
Chromatography Examples For each example, the smaller, green solute is more strongly retained than the larger, red solute. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography is a method by which a mixture is separated by distributing its components between two phases. chromatography is a group of laboratory techniques used to separate the. Chromatography Examples.
From www.slideshare.net
Principles and application of chromatography Chromatography Examples four examples of interactions between a solute and the stationary phase: Learn more about chromatography in this article. The stationary phase remains fixed in place while the mobile phase carries the components. chromatography is a method by which a mixture is separated by distributing its components between two phases. For each example, the smaller, green solute is more. Chromatography Examples.
From
Chromatography Examples chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture. chromatography is. Chromatography Examples.