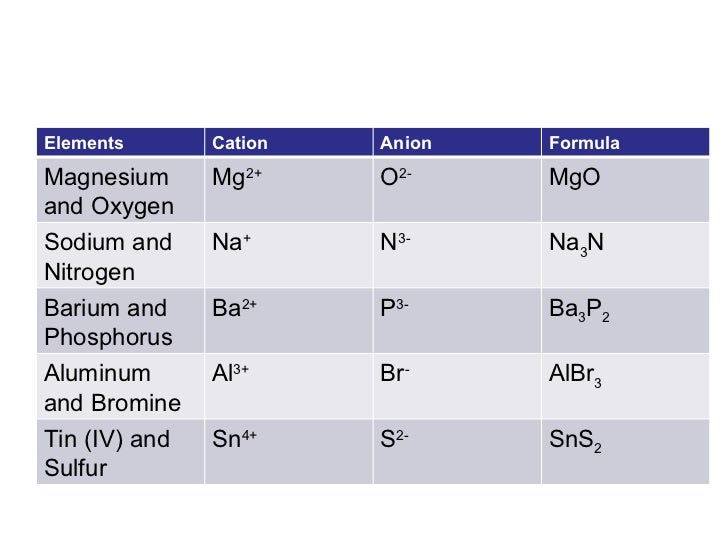
Mg Br Ionic Compound Formula . Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Write the chemical formula for an ionic compound composed of each pair of ions. Then, identify the anion and write down. The magnesium ion and the carbonate ion; Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Magnesium and bromine are used as an example. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^.
from www.slideshare.net
When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The magnesium ion and the carbonate ion; To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Magnesium and bromine are used as an example. Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Write the chemical formula for an ionic compound composed of each pair of ions. Then, identify the anion and write down. Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule.
Ionic bonding binary
Mg Br Ionic Compound Formula Magnesium and bromine are used as an example. Magnesium and bromine are used as an example. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The magnesium ion and the carbonate ion; Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Write the chemical formula for an ionic compound composed of each pair of ions. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Then, identify the anion and write down.
From studylib.net
Writing Formulas for Ionic Compounds Mg Br Ionic Compound Formula Magnesium and bromine are used as an example. Write the chemical formula for an ionic compound composed of each pair of ions. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Learn how to figure out the formulas of ionic compounds, including the handy crisscross. Mg Br Ionic Compound Formula.
From learninglibrarywood88.z19.web.core.windows.net
How To Write Ionic Formulas Mg Br Ionic Compound Formula Write the chemical formula for an ionic compound composed of each pair of ions. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Magnesium and bromine. Mg Br Ionic Compound Formula.
From www.slideserve.com
PPT ION FORMATION PowerPoint Presentation, free download ID5668219 Mg Br Ionic Compound Formula A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The magnesium ion and the carbonate ion; Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Write the chemical formula for an ionic compound composed of. Mg Br Ionic Compound Formula.
From studylib.net
IONIC COMPOUNDS Formulas & Nomenclature Mg Br Ionic Compound Formula Write the chemical formula for an ionic compound composed of each pair of ions. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Then, identify the anion and write down.. Mg Br Ionic Compound Formula.
From worksheetzonemasters.z19.web.core.windows.net
Formulas For Ionic Compounds Practice Mg Br Ionic Compound Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Then, identify the anion and write down. Write the chemical formula for an ionic compound composed of each pair of ions. To find the formula of an ionic compound, first identify the cation and write down. Mg Br Ionic Compound Formula.
From www.coursehero.com
[Solved] Fill in the name and empirical formula of each ionic compound that... Course Hero Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Write the chemical formula for an ionic compound composed of each pair of ions. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Then, identify the anion and write down. The magnesium ion and the carbonate ion; To find the formula. Mg Br Ionic Compound Formula.
From studylib.net
Ionic Compounds Naming Mg Br Ionic Compound Formula The magnesium ion and the carbonate ion; Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down. Magnesium bromide is a white crystalline ionic compound with a chemical formula. Mg Br Ionic Compound Formula.
From www.numerade.com
SOLVED Write the formula for the ionic compound formed from each pair of elements. sodium and Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The magnesium ion and the carbonate ion; When an ionic compound is formed. Mg Br Ionic Compound Formula.
From joinsvqac.blob.core.windows.net
Bromine And Magnesium Formula at Harold Rust blog Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium and bromine are. Mg Br Ionic Compound Formula.
From www.numerade.com
SOLVEDChemistry Fundamentals and Principles Damidsicn presented by Macmillan Learning Write Mg Br Ionic Compound Formula Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Magnesium and bromine are used as an example. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. To find the formula of an ionic compound, first identify the cation and. Mg Br Ionic Compound Formula.
From chemistry291.blogspot.com
What Is the Magnesium Bromide Formula? Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the chemical formula for an ionic compound. Mg Br Ionic Compound Formula.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free download ID2962564 Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Then, identify the anion and write down. Write the chemical formula for an ionic compound composed of each pair of ions. The magnesium. Mg Br Ionic Compound Formula.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free download ID2962564 Mg Br Ionic Compound Formula To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Write the chemical formula for an ionic compound composed of each pair of ions. Magnesium and bromine are used as an example. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Learn how to figure out the formulas of ionic compounds,. Mg Br Ionic Compound Formula.
From www.slideshare.net
Ionic bonding binary Mg Br Ionic Compound Formula Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. The magnesium ion and the carbonate ion; When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Write. Mg Br Ionic Compound Formula.
From worksheetlistket.z19.web.core.windows.net
How To Write Formulas Of Ionic Compounds Mg Br Ionic Compound Formula A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. When an ionic compound. Mg Br Ionic Compound Formula.
From nzohomeworkuht.web.fc2.com
How to write ionic formulas for compounds Mg Br Ionic Compound Formula To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The magnesium ion and the carbonate ion; Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Write the chemical formula for an ionic compound composed of each pair of ions. Learn how to figure out the formulas. Mg Br Ionic Compound Formula.
From study.com
Chemical Formula for Ionic Compound Binary & Polyatomic Lesson Mg Br Ionic Compound Formula The magnesium ion and the carbonate ion; Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Learn how to figure out. Mg Br Ionic Compound Formula.
From www.showme.com
ionic bonding (Al & Br) Science ShowMe Mg Br Ionic Compound Formula Write the chemical formula for an ionic compound composed of each pair of ions. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. The magnesium ion and the carbonate ion; To find the formula of an ionic compound, first identify the cation and write down. Mg Br Ionic Compound Formula.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free download ID2962564 Mg Br Ionic Compound Formula Then, identify the anion and write down. Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Magnesium and bromine are used as an example. The magnesium ion and the carbonate ion; When an ionic compound is formed from magnesium and oxygen,. Mg Br Ionic Compound Formula.
From www.slideserve.com
PPT ION FORMATION PowerPoint Presentation, free download ID5668219 Mg Br Ionic Compound Formula Magnesium and bromine are used as an example. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Write the chemical formula for an ionic compound composed of each pair of ions. Learn how to figure out. Mg Br Ionic Compound Formula.
From www.youtube.com
Ionic compounds and its formula MgBr2 YouTube Mg Br Ionic Compound Formula A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Learn how to figure out the formulas of. Mg Br Ionic Compound Formula.
From slideplayer.com
Chemical Compounds Chapter ppt download Mg Br Ionic Compound Formula A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Magnesium and bromine are used as an example. Then, identify the anion and write down. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. To find the formula of an. Mg Br Ionic Compound Formula.
From www.youtube.com
How to Write the Net Ionic Equation for HBr + Mg(OH)2 = MgBr2 + H2O YouTube Mg Br Ionic Compound Formula Magnesium and bromine are used as an example. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Then, identify the anion and write down. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). To find the formula of an ionic compound,. Mg Br Ionic Compound Formula.
From www.studypool.com
SOLUTION Chemical formula and naming of ionic compound Studypool Mg Br Ionic Compound Formula The magnesium ion and the carbonate ion; When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Write. Mg Br Ionic Compound Formula.
From www.slideshare.net
Ionic Bonds Chapter 7 Mg Br Ionic Compound Formula Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. To find the formula of an ionic compound, first identify the cation. Mg Br Ionic Compound Formula.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + HBr = MgBr2 + H2 YouTube Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Explore the properties, synthesis,. Mg Br Ionic Compound Formula.
From exodpglyd.blob.core.windows.net
Magnesium Bromide Chemical Equation at Mario Kemper blog Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Then, identify the anion and write down. Write the chemical formula for an ionic compound composed of each pair of ions. The magnesium ion and the carbonate ion; Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. A magnesium cation (\mg^{2+}\). Mg Br Ionic Compound Formula.
From www.studypool.com
SOLUTION Chemical formula and naming of ionic compound Studypool Mg Br Ionic Compound Formula Write the chemical formula for an ionic compound composed of each pair of ions. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Then, identify the anion and write down. Magnesium and bromine are used as. Mg Br Ionic Compound Formula.
From www.youtube.com
How to Balance Mg + Br2 = MgBr2 (Magnesium + Bromine gas) YouTube Mg Br Ionic Compound Formula Then, identify the anion and write down. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. The magnesium ion and the. Mg Br Ionic Compound Formula.
From brainly.com
Using the model, write the chemical formula for an ionic compound that has Mg and Cl. Mg Br Ionic Compound Formula The magnesium ion and the carbonate ion; Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the chemical formula for an ionic. Mg Br Ionic Compound Formula.
From courses.lumenlearning.com
3.4 Ionic Nomenclature The Basics of General, Organic, and Biological Chemistry Mg Br Ionic Compound Formula Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Then, identify the anion and write down. A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. The magnesium ion and the carbonate ion; To find the formula of an ionic compound, first. Mg Br Ionic Compound Formula.
From www.youtube.com
Writing Chemical Formulas For Ionic Compounds YouTube Mg Br Ionic Compound Formula To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Magnesium and bromine are used as an example. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the chemical formula for an ionic compound composed. Mg Br Ionic Compound Formula.
From will-blogstevens.blogspot.com
Which of the Following Represent the Lewis Structure for Mg Mg Br Ionic Compound Formula A magnesium cation (\mg^{2+}\) and two bromide anions \(br^. The magnesium ion and the carbonate ion; To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Magnesium bromide is a white crystalline ionic compound with a chemical formula \(mgbr_2\). Write the chemical formula for an ionic compound composed of each pair. Mg Br Ionic Compound Formula.
From www.youtube.com
How to Draw the Lewis Dot Structure for MgBr2 Magnesium bromide YouTube Mg Br Ionic Compound Formula The magnesium ion and the carbonate ion; Then, identify the anion and write down. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Write the chemical formula for an ionic compound composed of each pair of ions. Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. Magnesium bromide. Mg Br Ionic Compound Formula.
From www.sliderbase.com
Formula of Ionic Compounds Mg Br Ionic Compound Formula Explore the properties, synthesis, applications, and safety measures of magnesium bromide, an essential chemical compound. The magnesium ion and the carbonate ion; Magnesium and bromine are used as an example. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Then, identify the anion and write. Mg Br Ionic Compound Formula.