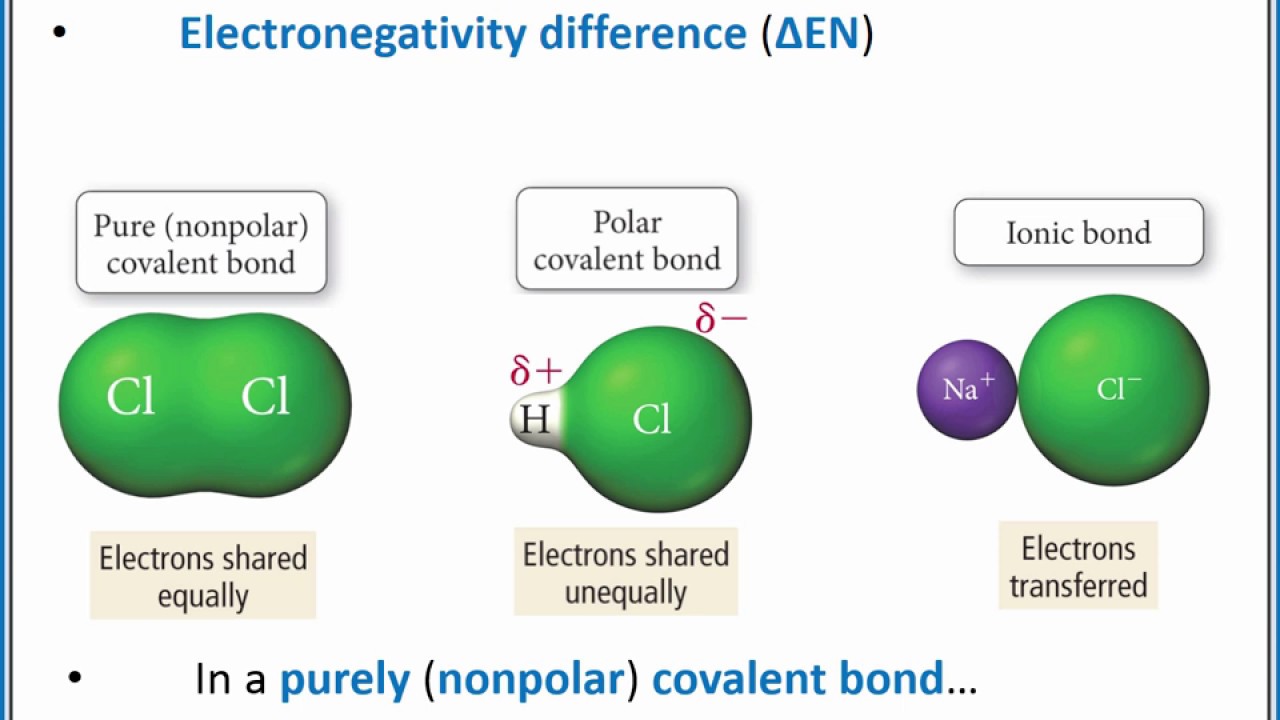
What Is Polar Nonpolar And Ionic . A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Ionic bonds are highly polar. The absolute values of the electronegativity differences between the atoms in the bonds. When it is large, the bond is polar covalent or ionic. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. However, there are nonpolar molecules with polar bonds and polar. Electronegativity is a measure of the tendency of an atom to. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. The negative charge of electrons balances the positive charge of protons in an atom. The electrons symmetrically distributed around the. Often, the polarity of the bonds is the same as the polarity of the molecule.
from www.youtube.com
The electrons symmetrically distributed around the. The negative charge of electrons balances the positive charge of protons in an atom. Electronegativity is a measure of the tendency of an atom to. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Often, the polarity of the bonds is the same as the polarity of the molecule. The absolute values of the electronegativity differences between the atoms in the bonds. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. However, there are nonpolar molecules with polar bonds and polar. When it is large, the bond is polar covalent or ionic.
CHEMISTRY 101 Using electronegativity to classify bonds as polar covalent, covalent, or ionic
What Is Polar Nonpolar And Ionic The absolute values of the electronegativity differences between the atoms in the bonds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Often, the polarity of the bonds is the same as the polarity of the molecule. The negative charge of electrons balances the positive charge of protons in an atom. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electrons symmetrically distributed around the. However, there are nonpolar molecules with polar bonds and polar. When it is large, the bond is polar covalent or ionic. Ionic bonds are highly polar. Electronegativity is a measure of the tendency of an atom to. The absolute values of the electronegativity differences between the atoms in the bonds. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity.
From simp-link.com
Difference between polar and nonpolar examples What Is Polar Nonpolar And Ionic However, there are nonpolar molecules with polar bonds and polar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. The electrons symmetrically distributed around the. The absolute values of the electronegativity differences between the atoms in the bonds. Electronegativity is a measure of the tendency of an atom to. Often,. What Is Polar Nonpolar And Ionic.
From physvids.com
Polar Covalent Bonds Clearly Explained for Easy Learning What Is Polar Nonpolar And Ionic Often, the polarity of the bonds is the same as the polarity of the molecule. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. When it is large, the bond is polar covalent or ionic. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the. What Is Polar Nonpolar And Ionic.
From www.windward.solutions
How does a polar bond differ from a covalent bond What Is Polar Nonpolar And Ionic The negative charge of electrons balances the positive charge of protons in an atom. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to. Often, the polarity of the bonds is the same as the polarity of the molecule. Ionic. What Is Polar Nonpolar And Ionic.
From www.slideserve.com
PPT Covalent Bonds Electronegativity differences and ionic/polar/nonpolar classification What Is Polar Nonpolar And Ionic Often, the polarity of the bonds is the same as the polarity of the molecule. Electronegativity is a measure of the tendency of an atom to. When it is large, the bond is polar covalent or ionic. The negative charge of electrons balances the positive charge of protons in an atom. The absolute values of the electronegativity differences between the. What Is Polar Nonpolar And Ionic.
From www.slideserve.com
PPT Covalent Bonds Electronegativity differences and ionic/polar/nonpolar classification What Is Polar Nonpolar And Ionic The negative charge of electrons balances the positive charge of protons in an atom. When it is large, the bond is polar covalent or ionic. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electrons symmetrically distributed around the. However, there are nonpolar molecules with polar bonds. What Is Polar Nonpolar And Ionic.
From www.youtube.com
Polar Covalent Bonds and Nonpolar Covalent bonds, Ionic Bonding Types of Chemical Bonds YouTube What Is Polar Nonpolar And Ionic Electronegativity is a measure of the tendency of an atom to. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. The electrons symmetrically distributed around the. Ionic bonds are highly polar.. What Is Polar Nonpolar And Ionic.
From chemicoolchemistry.tumblr.com
Electronegativity and Polarity cheMYSTERY What Is Polar Nonpolar And Ionic The electrons symmetrically distributed around the. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Often, the polarity of the bonds is the same as the polarity of the molecule. Ionic bonds are highly polar. When it is large, the bond is polar covalent or ionic. Whether a bond is. What Is Polar Nonpolar And Ionic.
From mungfali.com
Polar Covalent Bond Electronegativity What Is Polar Nonpolar And Ionic Ionic bonds are highly polar. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electrons symmetrically distributed around the. The negative charge of electrons balances the positive charge. What Is Polar Nonpolar And Ionic.
From www.slideserve.com
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free download ID988207 What Is Polar Nonpolar And Ionic The negative charge of electrons balances the positive charge of protons in an atom. The absolute values of the electronegativity differences between the atoms in the bonds. Electronegativity is a measure of the tendency of an atom to. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Often,. What Is Polar Nonpolar And Ionic.
From www.youtube.com
CHEMISTRY 101 Using electronegativity to classify bonds as polar covalent, covalent, or ionic What Is Polar Nonpolar And Ionic The absolute values of the electronegativity differences between the atoms in the bonds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. When it is large, the bond is polar covalent. What Is Polar Nonpolar And Ionic.
From sciencenotes.org
Polar and Nonpolar Molecules What Is Polar Nonpolar And Ionic When it is large, the bond is polar covalent or ionic. However, there are nonpolar molecules with polar bonds and polar. Ionic bonds are highly polar. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Whether a bond is nonpolar or polar covalent is determined by a property. What Is Polar Nonpolar And Ionic.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps What Is Polar Nonpolar And Ionic Ionic bonds are highly polar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electrons symmetrically distributed around the. Whether a bond is nonpolar or polar covalent is. What Is Polar Nonpolar And Ionic.
From www.chemistrylearner.com
Polar Covalent Bond Definition and Examples What Is Polar Nonpolar And Ionic Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. The negative charge of electrons balances the positive charge of protons in an atom. However, there are nonpolar molecules with. What Is Polar Nonpolar And Ionic.
From www.slideserve.com
PPT Covalent Bonds Electronegativity differences and ionic/polar/nonpolar classification What Is Polar Nonpolar And Ionic The absolute values of the electronegativity differences between the atoms in the bonds. Often, the polarity of the bonds is the same as the polarity of the molecule. Ionic bonds are highly polar. The negative charge of electrons balances the positive charge of protons in an atom. A bond in which the electronegativity difference between the atoms is between 0.5. What Is Polar Nonpolar And Ionic.
From www.youtube.com
Polar, NonPolar, and Ionic Compounds Explanation, Examples, and Practice YouTube What Is Polar Nonpolar And Ionic The electrons symmetrically distributed around the. The negative charge of electrons balances the positive charge of protons in an atom. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond.. What Is Polar Nonpolar And Ionic.
From www.youtube.com
Difference in Electronegativity part 3 How to identify polar bond ,non polar bond and ionic What Is Polar Nonpolar And Ionic When it is large, the bond is polar covalent or ionic. The electrons symmetrically distributed around the. The negative charge of electrons balances the positive charge of protons in an atom. Electronegativity is a measure of the tendency of an atom to. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to. What Is Polar Nonpolar And Ionic.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Is Polar Nonpolar And Ionic However, there are nonpolar molecules with polar bonds and polar. When it is large, the bond is polar covalent or ionic. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to. Often, the polarity of the bonds is the same. What Is Polar Nonpolar And Ionic.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Is Polar Nonpolar And Ionic Ionic bonds are highly polar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Often, the polarity of the bonds is the same as the polarity of the molecule. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms. What Is Polar Nonpolar And Ionic.
From www.slideserve.com
PPT Covalent Bonds Electronegativity differences and ionic/polar/nonpolar classification What Is Polar Nonpolar And Ionic However, there are nonpolar molecules with polar bonds and polar. The absolute values of the electronegativity differences between the atoms in the bonds. Electronegativity is a measure of the tendency of an atom to. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. The electrons symmetrically distributed around the. Calculate. What Is Polar Nonpolar And Ionic.
From nursehub.com
Understanding Types of Chemical Bonds NurseHub What Is Polar Nonpolar And Ionic Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Often, the polarity of the bonds is the same as the polarity of the molecule. Ionic bonds are highly polar. The electrons symmetrically distributed around the. Whether a bond is nonpolar or polar covalent is determined by a property. What Is Polar Nonpolar And Ionic.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Is Polar Nonpolar And Ionic Ionic bonds are highly polar. When it is large, the bond is polar covalent or ionic. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. The electrons symmetrically distributed around the. Electronegativity is a measure of the tendency of an atom to. Calculate the electronegativity difference (δen) and average (en). What Is Polar Nonpolar And Ionic.
From msjschemclass.blogspot.com
Ms J's Chemistry Class Polar vs NonPolar Covalent Bonds What Is Polar Nonpolar And Ionic A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. The electrons symmetrically distributed around the. When it is large, the bond is polar covalent or ionic. However, there are nonpolar molecules with polar bonds and polar. Ionic bonds are highly polar. The negative charge of electrons balances the positive charge. What Is Polar Nonpolar And Ionic.
From www.youtube.com
Electronegativity and Chemical Bond Types (Covalent Polar, Covalent NonPolar, Ionic Bonds What Is Polar Nonpolar And Ionic The negative charge of electrons balances the positive charge of protons in an atom. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. However, there are nonpolar molecules with polar bonds and polar. The absolute values of the electronegativity differences between the atoms in the bonds. When it. What Is Polar Nonpolar And Ionic.
From simp-link.com
Difference between polar and nonpolar examples What Is Polar Nonpolar And Ionic Ionic bonds are highly polar. However, there are nonpolar molecules with polar bonds and polar. The absolute values of the electronegativity differences between the atoms in the bonds. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Whether a bond is nonpolar or polar covalent is determined by. What Is Polar Nonpolar And Ionic.
From studylib.net
Polar and Nonpolar Molecules What Is Polar Nonpolar And Ionic Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The absolute values of the electronegativity differences between the atoms in the bonds. However, there are nonpolar molecules with polar bonds and polar. Electronegativity is a measure of the tendency of an atom to. The electrons symmetrically distributed around. What Is Polar Nonpolar And Ionic.
From www.slideserve.com
PPT Electronegativity and Polarity PowerPoint Presentation, free download ID1486756 What Is Polar Nonpolar And Ionic Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. The absolute values of the electronegativity differences between the atoms in the bonds. The negative charge of electrons balances the positive charge of protons in an atom. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use. What Is Polar Nonpolar And Ionic.
From psiberg.com
Polar vs Nonpolar bonds What is the Main Difference? PSIBERG What Is Polar Nonpolar And Ionic The electrons symmetrically distributed around the. The absolute values of the electronegativity differences between the atoms in the bonds. The negative charge of electrons balances the positive charge of protons in an atom. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Calculate the electronegativity difference (δen) and average (en). What Is Polar Nonpolar And Ionic.
From www.youtube.com
Polar and Nonpolar Molecules YouTube What Is Polar Nonpolar And Ionic The absolute values of the electronegativity differences between the atoms in the bonds. When it is large, the bond is polar covalent or ionic. Often, the polarity of the bonds is the same as the polarity of the molecule. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. However, there. What Is Polar Nonpolar And Ionic.
From pediaa.com
Difference Between Polar and Nonpolar Molecules Definition, Formation, Properties, Examples What Is Polar Nonpolar And Ionic Ionic bonds are highly polar. The negative charge of electrons balances the positive charge of protons in an atom. When it is large, the bond is polar covalent or ionic. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Often, the polarity of the bonds is the same. What Is Polar Nonpolar And Ionic.
From surfguppy.com
Comparision of Bonds Surfguppy Chemistry made easy for visual learners What Is Polar Nonpolar And Ionic The negative charge of electrons balances the positive charge of protons in an atom. The absolute values of the electronegativity differences between the atoms in the bonds. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Whether a bond is nonpolar or polar covalent is determined by a. What Is Polar Nonpolar And Ionic.
From simp-link.com
Difference between polar and nonpolar examples What Is Polar Nonpolar And Ionic Electronegativity is a measure of the tendency of an atom to. The negative charge of electrons balances the positive charge of protons in an atom. The absolute values of the electronegativity differences between the atoms in the bonds. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. When it is. What Is Polar Nonpolar And Ionic.
From stilleducation.com
Polar and Nonpolar Covalent Bonds Characteristics & Differences Still Education What Is Polar Nonpolar And Ionic The absolute values of the electronegativity differences between the atoms in the bonds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electrons symmetrically distributed around the. However,. What Is Polar Nonpolar And Ionic.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Is Polar Nonpolar And Ionic A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar. The negative charge of electrons balances the positive charge of protons in an atom. However, there are nonpolar molecules with polar bonds and polar. When it is large, the bond is polar covalent or ionic. Often, the polarity of the bonds. What Is Polar Nonpolar And Ionic.
From www.britannica.com
polarity Definition & Examples Britannica What Is Polar Nonpolar And Ionic Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electrons symmetrically distributed around the. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Ionic bonds are highly polar. Often, the polarity of the bonds is the same. What Is Polar Nonpolar And Ionic.
From www.pinterest.ph
Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding, Covalent Bonding What Is Polar Nonpolar And Ionic Ionic bonds are highly polar. Electronegativity is a measure of the tendency of an atom to. When it is large, the bond is polar covalent or ionic. The negative charge of electrons balances the positive charge of protons in an atom. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine. What Is Polar Nonpolar And Ionic.