Spill Control Neutralization Reactions Can Release . Examples include sodium hydroxide, potassium hydroxide, and ammonia. B) sprinkle solid naoh on the spill. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. use a weak acid to neutralize bases. what should you do if you spill sulfuric acid on the countertop? if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. A) neutralize the acid with vinegar. develop a spill control procedure. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,.
from dewwool.com
Examples include sodium hydroxide, potassium hydroxide, and ammonia. B) sprinkle solid naoh on the spill. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. develop a spill control procedure. A) neutralize the acid with vinegar. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. what should you do if you spill sulfuric acid on the countertop? the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. use a weak acid to neutralize bases. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent.
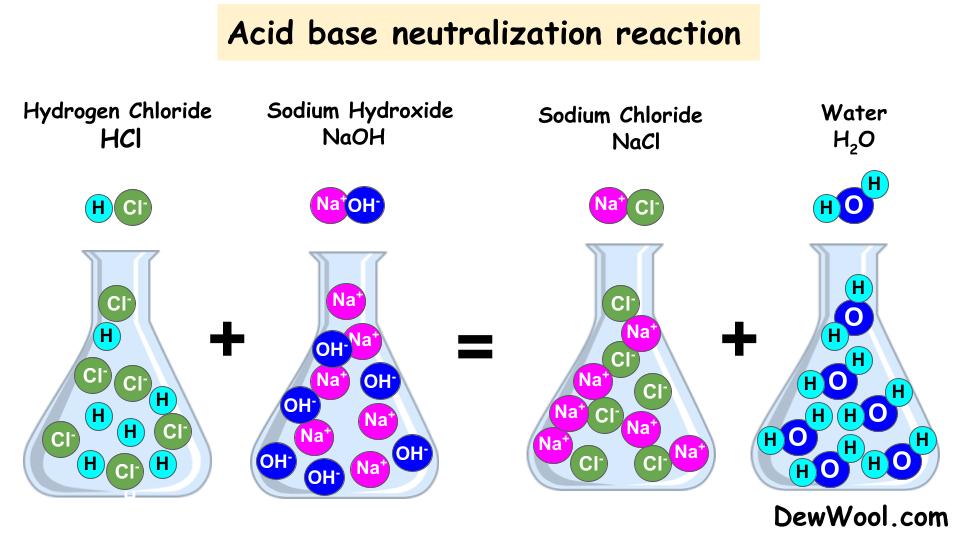
Neutralization ReactionDefinitionExamplesTypes DewWool
Spill Control Neutralization Reactions Can Release if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. what should you do if you spill sulfuric acid on the countertop? the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. use a weak acid to neutralize bases. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. A) neutralize the acid with vinegar. Examples include sodium hydroxide, potassium hydroxide, and ammonia. develop a spill control procedure. B) sprinkle solid naoh on the spill.
From www.abcworksheet.com
What is a Neutralization Reaction Definition Spill Control Neutralization Reactions Can Release what should you do if you spill sulfuric acid on the countertop? the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. use a weak acid to neutralize bases. B) sprinkle solid naoh on the spill. Examples include sodium hydroxide, potassium hydroxide, and ammonia. if a spilled chemical is not. Spill Control Neutralization Reactions Can Release.
From www.chemicals.co.uk
Chemistry GCSE Revision Energy Changes Spill Control Neutralization Reactions Can Release the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. what should you do if you spill sulfuric acid on the countertop? A) neutralize the acid with vinegar. use a weak acid to neutralize bases. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful. Spill Control Neutralization Reactions Can Release.
From spillcontainment.com
Spill Containment Best Practices Spill Control Neutralization Reactions Can Release A) neutralize the acid with vinegar. develop a spill control procedure. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. chemical neutralization involves introducing a reactive substance to. Spill Control Neutralization Reactions Can Release.
From www.slideserve.com
PPT Properties of Solutions PowerPoint Presentation, free download Spill Control Neutralization Reactions Can Release silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. A) neutralize the acid with vinegar. B) sprinkle solid naoh on the spill. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. develop a spill control procedure. use a weak acid. Spill Control Neutralization Reactions Can Release.
From www.studypool.com
SOLUTION 5 4 types of neutralization reaction filled in slides Studypool Spill Control Neutralization Reactions Can Release chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. A) neutralize the acid with vinegar. develop a spill control procedure. B) sprinkle solid naoh on the spill. Examples include sodium hydroxide, potassium hydroxide, and. Spill Control Neutralization Reactions Can Release.
From www.youtube.com
10 class chemistry,neutralisation reaction//neutralization reaction by Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. use a weak acid to neutralize bases. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. what should you do if you spill sulfuric acid on the countertop? A) neutralize the acid with vinegar. the developed beads could slowly release the neutralizing. Spill Control Neutralization Reactions Can Release.
From www.chemicals.co.uk
What is Neutralisation in Chemistry? The Chemistry Blog Spill Control Neutralization Reactions Can Release Examples include sodium hydroxide, potassium hydroxide, and ammonia. develop a spill control procedure. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. A) neutralize the acid with vinegar. use a weak acid to neutralize bases. B) sprinkle solid naoh on the spill. chemical neutralization involves introducing a. Spill Control Neutralization Reactions Can Release.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1751482 Spill Control Neutralization Reactions Can Release use a weak acid to neutralize bases. develop a spill control procedure. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. if a spilled chemical is not hazardous, its cleanup (without the. Spill Control Neutralization Reactions Can Release.
From www.teachoo.com
[Class 7] Neutralisation Reaction Concept, Formula Teachoo Spill Control Neutralization Reactions Can Release silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. B) sprinkle solid naoh on the spill. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response. Spill Control Neutralization Reactions Can Release.
From slideplayer.com
Neutralization reactions ppt download Spill Control Neutralization Reactions Can Release A) neutralize the acid with vinegar. what should you do if you spill sulfuric acid on the countertop? silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. use a weak acid to neutralize bases. the developed beads could slowly release the neutralizing agent, thereby improving control of. Spill Control Neutralization Reactions Can Release.
From studylib.net
Neutralization Reactions Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. what should you do if you spill sulfuric acid on the countertop? if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. Examples include sodium. Spill Control Neutralization Reactions Can Release.
From printablekolimbaxa.z22.web.core.windows.net
How To Complete Neutralization Reactions Spill Control Neutralization Reactions Can Release what should you do if you spill sulfuric acid on the countertop? A) neutralize the acid with vinegar. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. the developed beads. Spill Control Neutralization Reactions Can Release.
From slideplayer.com
Neutralization Reactions ppt download Spill Control Neutralization Reactions Can Release A) neutralize the acid with vinegar. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. use a weak acid to neutralize bases. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. what should you do if you spill sulfuric acid on the. Spill Control Neutralization Reactions Can Release.
From ehs.princeton.edu
Chemical Spill Procedures Office of Environmental Health and Safety Spill Control Neutralization Reactions Can Release chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. B) sprinkle solid naoh on the spill. A) neutralize the acid with vinegar. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. Examples include sodium hydroxide, potassium hydroxide, and ammonia. use a weak acid. Spill Control Neutralization Reactions Can Release.
From dewwool.com
Neutralization ReactionDefinitionExamplesTypes DewWool Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. develop a spill control procedure. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. Examples include sodium hydroxide, potassium hydroxide, and ammonia. use a weak acid to neutralize bases. silicon is a major component of traditional spill control products. Spill Control Neutralization Reactions Can Release.
From exokrimdo.blob.core.windows.net
How Can Chemical Reactions Be Used To Help Clean Up Oil Spills at Mark Spill Control Neutralization Reactions Can Release the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. Examples include sodium hydroxide, potassium hydroxide, and ammonia. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. what should you do if you spill sulfuric acid on the countertop? use a. Spill Control Neutralization Reactions Can Release.
From www.youtube.com
Visualizing a Neutralization Reaction (HCl + NaOH) YouTube Spill Control Neutralization Reactions Can Release if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. what should you do if you spill sulfuric acid on the countertop? Examples include sodium hydroxide, potassium hydroxide, and ammonia. use a weak acid to neutralize bases. A) neutralize the acid with vinegar. develop a spill control. Spill Control Neutralization Reactions Can Release.
From slideplayer.com
Neutralization Reactions ppt download Spill Control Neutralization Reactions Can Release Examples include sodium hydroxide, potassium hydroxide, and ammonia. use a weak acid to neutralize bases. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. what should you do if you spill sulfuric acid on the countertop? B) sprinkle solid naoh on the spill. if a spilled chemical is not. Spill Control Neutralization Reactions Can Release.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID2244088 Spill Control Neutralization Reactions Can Release use a weak acid to neutralize bases. B) sprinkle solid naoh on the spill. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. Examples include sodium hydroxide, potassium hydroxide, and ammonia.. Spill Control Neutralization Reactions Can Release.
From www.coursehero.com
worksheet1 2.doc Laboratory AcidBase Neutralization Reactions The Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. use a weak acid to neutralize bases. what should you do if you spill sulfuric acid on the countertop? silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. develop a spill control procedure. the developed beads could slowly release. Spill Control Neutralization Reactions Can Release.
From www.youtube.com
Neutralization Reaction with Experiment Sodium hydroxide , Sulphuric Spill Control Neutralization Reactions Can Release develop a spill control procedure. Examples include sodium hydroxide, potassium hydroxide, and ammonia. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. use a weak acid to neutralize bases. . Spill Control Neutralization Reactions Can Release.
From www.youtube.com
Neutralization Reactions. (Chemistry Ch. 11, Part 3) YouTube Spill Control Neutralization Reactions Can Release chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. use a weak acid to neutralize bases. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. the developed beads could slowly release the neutralizing agent, thereby improving control of the. Spill Control Neutralization Reactions Can Release.
From www.youtube.com
Chemical Spill Response Training Video YouTube Spill Control Neutralization Reactions Can Release what should you do if you spill sulfuric acid on the countertop? chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. A) neutralize the acid with vinegar. develop a spill control procedure. use a weak acid to neutralize bases. B) sprinkle solid naoh on the spill. if a. Spill Control Neutralization Reactions Can Release.
From lessonlibrarybenempt.z21.web.core.windows.net
How To Solve Neutralization Reactions Spill Control Neutralization Reactions Can Release chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. what should you do if you spill sulfuric acid on the countertop? Examples include sodium hydroxide, potassium hydroxide, and ammonia. the developed. Spill Control Neutralization Reactions Can Release.
From ehs.washington.edu
Workplace EHS Spill Control Neutralization Reactions Can Release use a weak acid to neutralize bases. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts. Spill Control Neutralization Reactions Can Release.
From study.com
Predicting the Products of a Neutralization Reaction Chemistry Spill Control Neutralization Reactions Can Release the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. B) sprinkle solid naoh on the spill. use a weak acid to neutralize bases. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. what should you do if you spill sulfuric acid on. Spill Control Neutralization Reactions Can Release.
From mavink.com
Spill Response Flow Chart Spill Control Neutralization Reactions Can Release the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. B) sprinkle solid naoh on the spill. A) neutralize the acid with vinegar. what should you do if you spill sulfuric acid. Spill Control Neutralization Reactions Can Release.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. use a weak acid to neutralize bases. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. develop a spill control procedure. what should. Spill Control Neutralization Reactions Can Release.
From www.youtube.com
Neutralisation Reaction What is a neutralization reaction? YouTube Spill Control Neutralization Reactions Can Release A) neutralize the acid with vinegar. Examples include sodium hydroxide, potassium hydroxide, and ammonia. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. B) sprinkle solid naoh on the spill. silicon. Spill Control Neutralization Reactions Can Release.
From www.studocu.com
Activity 7 P1 Laboratory AcidBase Neutralization Reactions The Spill Control Neutralization Reactions Can Release the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. what should you do if you spill sulfuric acid on the countertop? silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. develop a spill control procedure. Examples include sodium hydroxide, potassium. Spill Control Neutralization Reactions Can Release.
From blog.storemasta.com.au
Chemical Spill Control The Complete Guide Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. the developed beads could slowly release the neutralizing agent, thereby improving control of the neutralization rate. what should you do if you spill sulfuric acid on the countertop? if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. Examples include sodium. Spill Control Neutralization Reactions Can Release.
From www.youtube.com
Neutralization reaction neutralization reaction class 10 YouTube Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. use a weak acid to neutralize bases. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. develop a spill control procedure.. Spill Control Neutralization Reactions Can Release.
From studiousguy.com
7 Neutralization Examples in Everyday Life StudiousGuy Spill Control Neutralization Reactions Can Release Examples include sodium hydroxide, potassium hydroxide, and ammonia. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. develop a spill control procedure. use a weak acid to neutralize bases. A) neutralize the acid with vinegar. chemical neutralization involves introducing a reactive substance to the spilled chemical to. Spill Control Neutralization Reactions Can Release.
From eepowersolutions.com
Battery Spill Containment & Neutralization Pillows Eagle Eye Power Spill Control Neutralization Reactions Can Release B) sprinkle solid naoh on the spill. if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. silicon is a major component of traditional spill control products (sand, vermiculite) and reacts adversely with hydrofluoric acid,. Examples include sodium hydroxide, potassium hydroxide, and ammonia. the developed beads could slowly. Spill Control Neutralization Reactions Can Release.
From www.teachoo.com
[Class 7] Neutralisation Reaction Concept, Formula Teachoo Spill Control Neutralization Reactions Can Release use a weak acid to neutralize bases. chemical neutralization involves introducing a reactive substance to the spilled chemical to counteract its harmful effects. what should you do if you spill sulfuric acid on the countertop? if a spilled chemical is not hazardous, its cleanup (without the assistance of an emergency response team) is dependent. develop. Spill Control Neutralization Reactions Can Release.