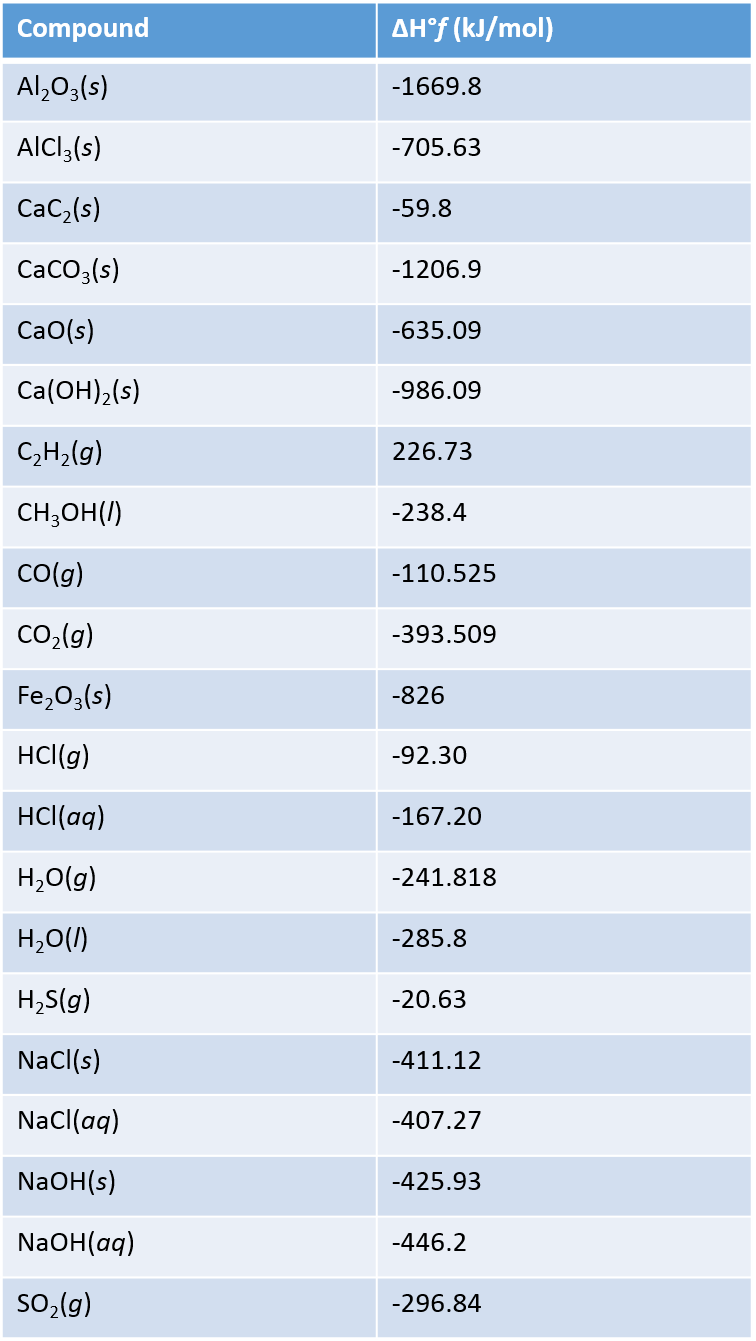
Standard Enthalpy Of Formation Mercury . 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation for an element in its standard state is zero!!!! a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Elements in their standard state are not.
from exokycsnc.blob.core.windows.net
the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is.
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog
Standard Enthalpy Of Formation Mercury a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation for an element in its standard state is zero!!!! 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; Elements in their standard state are not.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Mercury 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. by definition, the standard enthalpy of formation of an element in its most stable form is. Standard Enthalpy Of Formation Mercury.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation Mercury the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation Mercury.
From www.youtube.com
STANDARD ENTHALPY OF FORMATION THERMOCHEMICAL EQUATION Chemistry Standard Enthalpy Of Formation Mercury 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Standard Enthalpy Of Formation Mercury.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation for an element in its standard state is zero!!!! 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left. Standard Enthalpy Of Formation Mercury.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Standard Enthalpy Of Formation Mercury Elements in their standard state are not. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is. Standard Enthalpy Of Formation Mercury.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. Elements in their standard state are not. a standard enthalpy of formation $δh°_f$ is. Standard Enthalpy Of Formation Mercury.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Mercury the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Elements in their standard state are not. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or. Standard Enthalpy Of Formation Mercury.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign.. Standard Enthalpy Of Formation Mercury.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 Standard Enthalpy Of Formation Mercury 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole. Standard Enthalpy Of Formation Mercury.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation for an element in its standard state is zero!!!! 6c\left (s, graphite. Standard Enthalpy Of Formation Mercury.
From www.researchgate.net
Molecular weight, standard enthalpy of formation and thermal Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation for an element in its standard state. Standard Enthalpy Of Formation Mercury.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Mercury the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left. Standard Enthalpy Of Formation Mercury.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in. Standard Enthalpy Of Formation Mercury.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; the standard enthalpy of formation is given the symbol δfho δ. Standard Enthalpy Of Formation Mercury.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is given the symbol δfho δ f h. Standard Enthalpy Of Formation Mercury.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Mercury the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation Mercury.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Mercury 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Elements in their standard state are not. the standard enthalpy. Standard Enthalpy Of Formation Mercury.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation Mercury a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left. Standard Enthalpy Of Formation Mercury.
From www.researchgate.net
Formation enthalpy of the mercury complexes calcu lated in AM1 Standard Enthalpy Of Formation Mercury the standard enthalpy of formation for an element in its standard state is zero!!!! 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of. Standard Enthalpy Of Formation Mercury.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation for an element in its standard state is zero!!!! 6c\left (s, graphite \right. Standard Enthalpy Of Formation Mercury.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when. Standard Enthalpy Of Formation Mercury.
From www.scribd.com
Standard Enthalpy of Formation PDF Solvation Chemical Process Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of glucose from the elements at 25°c is the. Standard Enthalpy Of Formation Mercury.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Enthalpy Of Formation Mercury the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which. Standard Enthalpy Of Formation Mercury.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Elements in their standard state are not. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right. Standard Enthalpy Of Formation Mercury.
From dokumen.tips
(PDF) Standard Enthalpy of Formation* for Various Compoundsnshsscience Standard Enthalpy Of Formation Mercury Elements in their standard state are not. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: by definition, the standard enthalpy of. Standard Enthalpy Of Formation Mercury.
From www.researchgate.net
Formation enthalpy of the mercury complexes calcu lated in AM1 Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Elements in their standard state are not. 6c\left (s, graphite \right ) + 6h_ {2}\left. Standard Enthalpy Of Formation Mercury.
From www.researchgate.net
The calculated M index and the experimental standard enthalpy of Standard Enthalpy Of Formation Mercury 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; the standard enthalpy of formation for an element in its standard state is zero!!!! the. Standard Enthalpy Of Formation Mercury.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Mercury the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation for an element in its standard state is zero!!!! by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 193. Standard Enthalpy Of Formation Mercury.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the. Standard Enthalpy Of Formation Mercury.
From slideplayer.com
Standard Enthalpies of Formation ppt download Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.. Standard Enthalpy Of Formation Mercury.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation for an element in its standard state is zero!!!! Elements. Standard Enthalpy Of Formation Mercury.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. 6c\left (s, graphite \right ) + 6h_ {2}\left (g \right ) + 3o_ {2}\left (g \right ) \rightarrow c_ {6}h_ {12}o_ {6}\left (s \right )\; a standard enthalpy of formation $δh°_f$ is an enthalpy change for a. Standard Enthalpy Of Formation Mercury.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Mercury the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change. Standard Enthalpy Of Formation Mercury.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Elements in their standard state are not. the standard enthalpy of formation for an. Standard Enthalpy Of Formation Mercury.
From www.researchgate.net
The calculated standard enthalpy of formation at 25 °C in the CuSe Standard Enthalpy Of Formation Mercury by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Elements in their standard state are not. the standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Mercury.