Standard Enthalpy Of Formation Practice Problems . The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. ° indicates thermal energy (heat or temperature) The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. These are worked example problems calculating the heat of formation. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). (the molar enthalpy of formation,∆ * $, for h. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. To find the enthalpy of. Calculate δh° rxn for this reaction using standard enthalpies of formation.
from www.chegg.com
The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: Calculate δh° rxn for this reaction using standard enthalpies of formation. To find the enthalpy of. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. (the molar enthalpy of formation,∆ * $, for h. ° indicates thermal energy (heat or temperature) (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). These are worked example problems calculating the heat of formation.
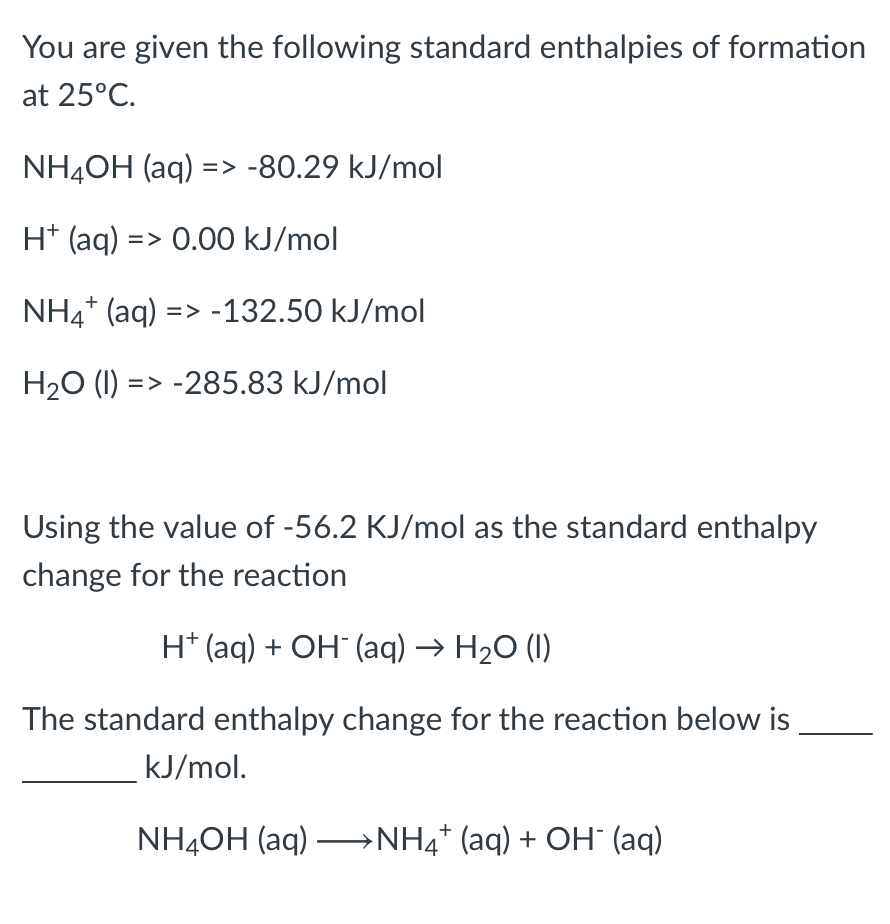
Solved You are given the following standard enthalpies of
Standard Enthalpy Of Formation Practice Problems Calculate δh° rxn for this reaction using standard enthalpies of formation. To find the enthalpy of. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). ° indicates thermal energy (heat or temperature) Calculate δh° rxn for this reaction using standard enthalpies of formation. These are worked example problems calculating the heat of formation. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: H indicates enthalpy, which is only measured as a change, not as an instantaneous value. The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. (the molar enthalpy of formation,∆ * $, for h. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements:
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Practice Problems These are worked example problems calculating the heat of formation. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: To find the enthalpy of. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). A) ∆calculate the standard enthalpy change, ’ $ (), for. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved You are given the following standard enthalpies of Standard Enthalpy Of Formation Practice Problems The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the. Standard Enthalpy Of Formation Practice Problems.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Practice Problems (the molar enthalpy of formation,∆ * $, for h. To find the enthalpy of. Calculate δh° rxn for this reaction using standard enthalpies of formation. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: These are worked example problems calculating the heat of formation. A). Standard Enthalpy Of Formation Practice Problems.
From printablemagicackermann.z13.web.core.windows.net
Enthalpy Practice Problems With Answers Standard Enthalpy Of Formation Practice Problems (the molar enthalpy of formation,∆ * $, for h. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: These are worked example problems calculating the heat of formation. °. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Practice Problems The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. (a) liquid (b) solid (c) gas (d). Standard Enthalpy Of Formation Practice Problems.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Practice Problems ° indicates thermal energy (heat or temperature) (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). Calculate δh° rxn for this reaction using standard enthalpies of formation. These are worked example problems calculating the heat of formation. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: H indicates enthalpy, which is. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved The standard enthalpy change for the following Standard Enthalpy Of Formation Practice Problems The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. These are worked example problems calculating the heat of formation. ° indicates thermal energy (heat or temperature) To find the enthalpy of. Calculate δh° rxn for this reaction using standard enthalpies. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved Calculate the Standard Enthalpy of Formation of NOCl Standard Enthalpy Of Formation Practice Problems The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: To find the enthalpy of. Calculate δh° rxn for this reaction using standard enthalpies of formation. (the molar enthalpy of formation,∆ * $, for h. A) ∆calculate the standard enthalpy change, ’ $ (), for the. Standard Enthalpy Of Formation Practice Problems.
From printablezonemcdonald.z21.web.core.windows.net
Enthalpy Practice Problems With Answers Pdf Standard Enthalpy Of Formation Practice Problems The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: To find the enthalpy of. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. These are worked example problems calculating the heat of formation. Calculate δh° rxn for this reaction. Standard Enthalpy Of Formation Practice Problems.
From printablelibraleigh.z21.web.core.windows.net
Enthalpy Practice Problems With Answers Pdf Standard Enthalpy Of Formation Practice Problems A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. To find the enthalpy of. Calculate δh° rxn for this reaction using standard enthalpies. Standard Enthalpy Of Formation Practice Problems.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Enthalpy Of Formation Practice Problems These are worked example problems calculating the heat of formation. The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. Practice problems answers (1). Standard Enthalpy Of Formation Practice Problems.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Practice Problems The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. (a) liquid (b) solid (c) gas (d). Standard Enthalpy Of Formation Practice Problems.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Standard Enthalpy Of Formation Practice Problems Calculate δh° rxn for this reaction using standard enthalpies of formation. (the molar enthalpy of formation,∆ * $, for h. To find the enthalpy of. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. These are worked example problems calculating the heat of formation. H indicates enthalpy, which is only measured as. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Practice Problems Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: To find the enthalpy of. These are worked example problems calculating the heat of formation. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). (the molar enthalpy of formation,∆ * $, for h. The symbol for the standard heat of formation (also. Standard Enthalpy Of Formation Practice Problems.
From www.numerade.com
SOLVEDFor which reaction the change of the enthalpy of the reaction Standard Enthalpy Of Formation Practice Problems (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). ° indicates thermal energy (heat or temperature) To find the enthalpy of. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: Calculate δh° rxn for this reaction using standard enthalpies of formation. These are. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Practice Problems ° indicates thermal energy (heat or temperature) Calculate δh° rxn for this reaction using standard enthalpies of formation. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: To find the enthalpy of. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. A) ∆calculate the standard enthalpy change,. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Practice Problems Calculate δh° rxn for this reaction using standard enthalpies of formation. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: These are worked example problems calculating the heat of formation. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. The enthalpy of formation, or standard enthalpy of. Standard Enthalpy Of Formation Practice Problems.
From priaxon.com
What Is Standard Enthalpy Of Formation Of Nh3 Gas Templates Printable Standard Enthalpy Of Formation Practice Problems These are worked example problems calculating the heat of formation. (the molar enthalpy of formation,∆ * $, for h. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: ° indicates thermal energy (heat or temperature) The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Practice Problems ° indicates thermal energy (heat or temperature) Calculate δh° rxn for this reaction using standard enthalpies of formation. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: A) ∆calculate the standard enthalpy change, ’ $ (), for the. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Practice Problems (the molar enthalpy of formation,∆ * $, for h. Calculate δh° rxn for this reaction using standard enthalpies of formation. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. ° indicates thermal energy (heat or temperature) These are worked example problems calculating the heat of formation. (a) liquid (b) solid (c) gas. Standard Enthalpy Of Formation Practice Problems.
From studylib.net
Enthalpy worksheets 1& 2 Standard Enthalpy Of Formation Practice Problems Calculate δh° rxn for this reaction using standard enthalpies of formation. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. These are worked example problems calculating the heat of formation. The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its. Standard Enthalpy Of Formation Practice Problems.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Practice Problems Calculate δh° rxn for this reaction using standard enthalpies of formation. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. ° indicates thermal energy (heat or temperature) Practice problems answers (1) identify the standard state (solid, liquid. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved Table 1. Standard enthalpies of formation for Standard Enthalpy Of Formation Practice Problems The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. (the molar enthalpy of formation,∆ * $, for h. Practice problems answers (1) identify the standard. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved A scientist measures the standard enthalpy change for Standard Enthalpy Of Formation Practice Problems H indicates enthalpy, which is only measured as a change, not as an instantaneous value. Calculate δh° rxn for this reaction using standard enthalpies of formation. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). The enthalpy of formation, or standard. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Practice Problems A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). Calculate δh° rxn for this reaction using standard enthalpies of formation. The symbol for the standard heat. Standard Enthalpy Of Formation Practice Problems.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Practice Problems The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f or δh f ° where: (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). H indicates enthalpy, which is only measured as a change, not as an instantaneous value. Calculate δh° rxn for this reaction using standard enthalpies of. Standard Enthalpy Of Formation Practice Problems.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Practice Problems (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). H indicates enthalpy, which is only measured as a change, not as an instantaneous value. A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. ° indicates thermal energy (heat or temperature) These are worked example problems calculating the heat of formation.. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Practice Problems A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. ° indicates thermal energy (heat or temperature) These are worked example problems calculating the heat of formation. Practice problems answers (1) identify the standard state (solid, liquid or. Standard Enthalpy Of Formation Practice Problems.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Practice Problems The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: ° indicates thermal energy (heat or temperature) (a) liquid (b) solid (c) gas (d) liquid. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
Standard Enthalpies of Formation Problem (Chemistry) YouTube Standard Enthalpy Of Formation Practice Problems A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). (the molar enthalpy of formation,∆ * $, for h. To find the enthalpy of. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
Gibbs Free Energy Equilibrium Constant, Enthalpy & Entropy Equations Standard Enthalpy Of Formation Practice Problems These are worked example problems calculating the heat of formation. Calculate δh° rxn for this reaction using standard enthalpies of formation. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. (the molar enthalpy of formation,∆ * $, for h. Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following. Standard Enthalpy Of Formation Practice Problems.
From lessonberginmistling.z21.web.core.windows.net
Enthalpy Practice Problems With Answers Pdf Standard Enthalpy Of Formation Practice Problems Practice problems answers (1) identify the standard state (solid, liquid or gas) for the following elements: A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. The enthalpy of formation, or standard enthalpy of formation, is the change. Standard Enthalpy Of Formation Practice Problems.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Practice Problems A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. H indicates enthalpy, which is only measured as a change, not as an instantaneous value. Calculate δh° rxn for this reaction using standard enthalpies of formation. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). The enthalpy of formation, or standard. Standard Enthalpy Of Formation Practice Problems.
From www.youtube.com
Standard enthalpy of formation example question YouTube Standard Enthalpy Of Formation Practice Problems A) ∆calculate the standard enthalpy change, ’ $ (), for the reaction represented by the equation above. (the molar enthalpy of formation,∆ * $, for h. These are worked example problems calculating the heat of formation. (a) liquid (b) solid (c) gas (d) liquid (e) solid (2). Practice problems answers (1) identify the standard state (solid, liquid or gas) for. Standard Enthalpy Of Formation Practice Problems.
From www.studypool.com
SOLUTION 5 CALCULATION OF ENTHALPY CHANGES OF REACTIONS USING Standard Enthalpy Of Formation Practice Problems These are worked example problems calculating the heat of formation. (the molar enthalpy of formation,∆ * $, for h. To find the enthalpy of. The enthalpy of formation, or standard enthalpy of formation, is the change in enthalpy when one mole of a substance is formed from its elements in their standard states. H indicates enthalpy, which is only measured. Standard Enthalpy Of Formation Practice Problems.