Silver Electrodes Electrolysis . Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Silver is below hydrogen in the reactivity series and so is discharged in preference to. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon, which is. In the figure, the anode consists of a silver electrode, shown on the left.
from www.vecteezy.com
When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. In the figure, the anode consists of a silver electrode, shown on the left. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. Silver is below hydrogen in the reactivity series and so is discharged in preference to. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon, which is.
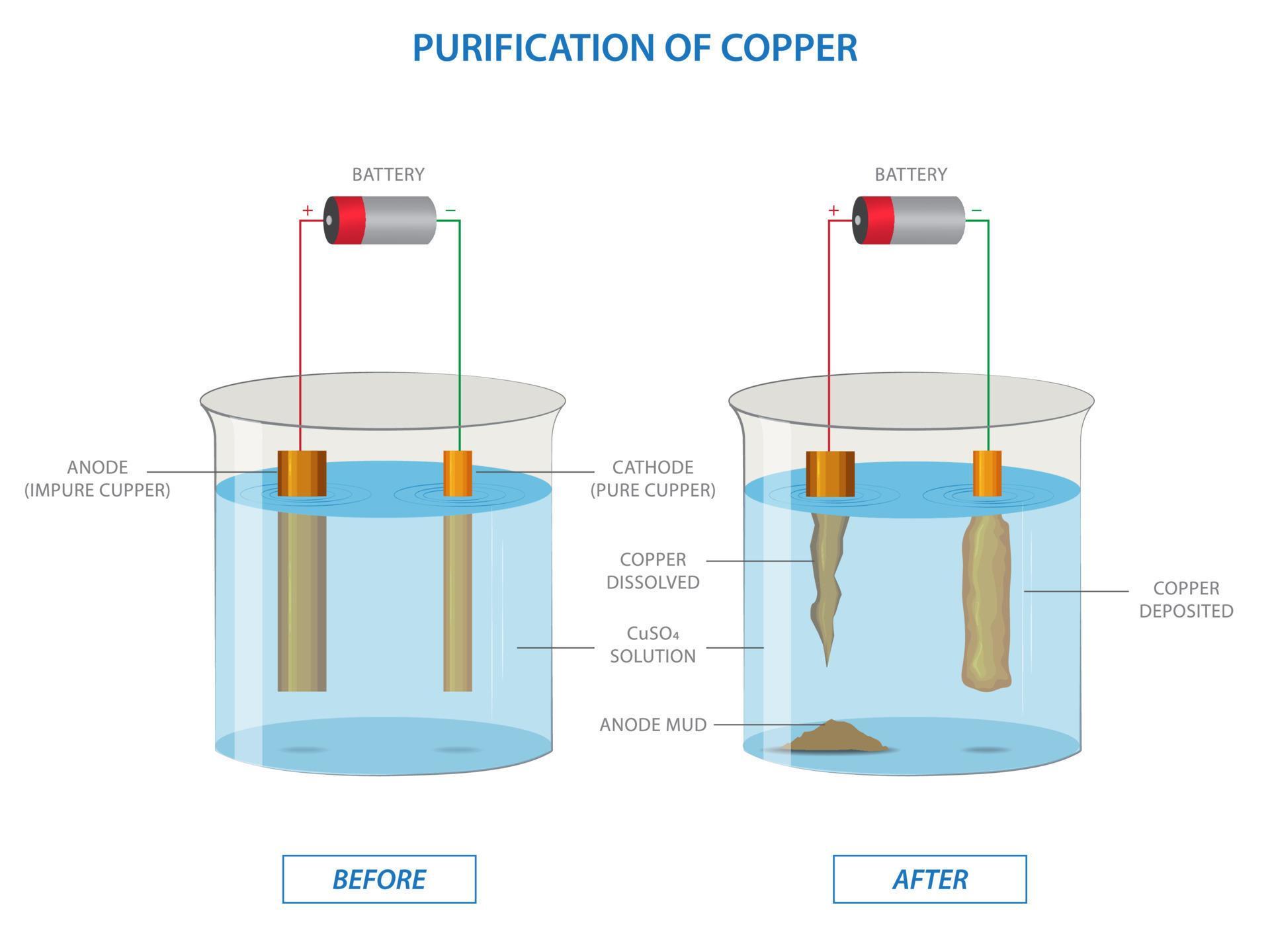
Electrolysis of copper sulfate solution with impure copper anode and
Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Silver is below hydrogen in the reactivity series and so is discharged in preference to. The cathode is located on the right and is the spoon, which is. Each silver atom loses one electron to form one. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. In the figure, the anode consists of a silver electrode, shown on the left. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and Silver Electrodes Electrolysis Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. In the figure, the anode consists of a silver electrode, shown on the left. Each silver atom loses one electron to form one. Silver is below hydrogen in the reactivity series and so is discharged in preference to. When we perform. Silver Electrodes Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Silver Electrodes Electrolysis Silver is below hydrogen in the reactivity series and so is discharged in preference to. Each silver atom loses one electron to form one. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. The cathode is located on the right and is the spoon, which is. The electrolysis of silver. Silver Electrodes Electrolysis.
From www.toppr.com
1. Ampic (2) 2.22 Predict the products of electrolysis in an aqueous Silver Electrodes Electrolysis The cathode is located on the right and is the spoon, which is. Silver is below hydrogen in the reactivity series and so is discharged in preference to. In the figure, the anode consists of a silver electrode, shown on the left. Each silver atom loses one electron to form one. When we perform electrolysis of say an aqueous solution. Silver Electrodes Electrolysis.
From saylordotorg.github.io
Electrochemistry Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon, which is. Silver is below hydrogen in the reactivity series and so is discharged in preference to. In the figure, the anode. Silver Electrodes Electrolysis.
From www.toppr.com
In the electrolysis of acidified AgNO_{3} solution using Ptelectrodes Silver Electrodes Electrolysis Silver is below hydrogen in the reactivity series and so is discharged in preference to. Each silver atom loses one electron to form one. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having. Silver Electrodes Electrolysis.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Silver Electrodes Electrolysis In the figure, the anode consists of a silver electrode, shown on the left. The cathode is located on the right and is the spoon, which is. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. Each silver atom loses one electron to form one. The electrolysis of silver nitrate. Silver Electrodes Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Silver Electrodes Electrolysis Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. The cathode. Silver Electrodes Electrolysis.
From brainly.in
[Solved] Explain electrolytic refining of copper Brainly.in Silver Electrodes Electrolysis When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. Each silver atom loses one electron to form one. The electrolysis of silver nitrate solution using inert electrodes at the. Silver Electrodes Electrolysis.
From www.youtube.com
Electrolysis of silver nitrate solution with carbon fibre electrode MVI Silver Electrodes Electrolysis The cathode is located on the right and is the spoon, which is. Each silver atom loses one electron to form one. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon. Silver Electrodes Electrolysis.
From www.youtube.com
Electrolysis of Copper Sulphate YouTube Silver Electrodes Electrolysis Silver is below hydrogen in the reactivity series and so is discharged in preference to. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. The cathode is located on the right and is the spoon, which is. When we perform electrolysis of say an aqueous solution of silver nitrate with. Silver Electrodes Electrolysis.
From favpng.com
Electrolytic Cell Electrolysis Silver Nitrate Galvanic Cell, PNG Silver Electrodes Electrolysis Silver is below hydrogen in the reactivity series and so is discharged in preference to. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon, which is. Here we report a nanoporous. Silver Electrodes Electrolysis.
From saylordotorg.github.io
Describing Electrochemical Cells Silver Electrodes Electrolysis Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon, which is. Silver is below hydrogen in the reactivity series and so is discharged in preference to. In the figure, the anode consists of a silver electrode, shown on the left. When we perform electrolysis of say an aqueous solution. Silver Electrodes Electrolysis.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Silver Electrodes Electrolysis When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Each silver atom loses one electron to form one. In the figure, the anode consists of a silver electrode, shown on the left. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to. Silver Electrodes Electrolysis.
From ceodjbtu.blob.core.windows.net
Water Electrolysis Engine at Heidi Rivera blog Silver Electrodes Electrolysis The cathode is located on the right and is the spoon, which is. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Here we report a nanoporous silver electrocatalyst that. Silver Electrodes Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Silver Electrodes Electrolysis Each silver atom loses one electron to form one. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Silver is below hydrogen in the reactivity series and so is discharged in preference to. The cathode is located on the right and is the spoon, which is. Here we report a nanoporous. Silver Electrodes Electrolysis.
From www.climate-policy-watcher.org
Electrolytic Silver Recovery Industrial Wastes Silver Electrodes Electrolysis When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Each silver atom loses one electron to form one. Silver is below hydrogen in the reactivity series and so is discharged in preference to. The cathode is located on the right and is the spoon, which is. In the. Silver Electrodes Electrolysis.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Silver Electrodes Electrolysis Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon, which is. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When. Silver Electrodes Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Silver Electrodes Electrolysis When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. The cathode is located on the right and is the spoon, which is. Silver is below hydrogen in the reactivity series. Silver Electrodes Electrolysis.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Silver Electrodes Electrolysis In the figure, the anode consists of a silver electrode, shown on the left. The cathode is located on the right and is the spoon, which is. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Silver is below hydrogen in the reactivity series and so is discharged in preference to.. Silver Electrodes Electrolysis.
From www.elixa.com
Colloidal Silver Electrodes Elixa Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. The cathode is located on the right and is the spoon, which is. Silver is below hydrogen in the reactivity series. Silver Electrodes Electrolysis.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Silver Electrodes Electrolysis When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon,. Silver Electrodes Electrolysis.
From paulaabbmathis.blogspot.com
Anode and Cathode in Electrolysis PaulaabbMathis Silver Electrodes Electrolysis When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon,. Silver Electrodes Electrolysis.
From www.youtube.com
Timelapse of copper sulfate electrolysis YouTube Silver Electrodes Electrolysis The cathode is located on the right and is the spoon, which is. Each silver atom loses one electron to form one. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Silver is below hydrogen in the reactivity series and so is discharged in preference to. In the figure, the anode. Silver Electrodes Electrolysis.
From byjus.com
7. Predict the products of electrolysis a)Aqueous solution of AgNO3 Silver Electrodes Electrolysis Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. In the figure, the anode consists of a silver electrode, shown on the left. Silver is below hydrogen in the reactivity series and. Silver Electrodes Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Silver is below hydrogen in the reactivity series and so is discharged in preference to. In the figure, the anode consists. Silver Electrodes Electrolysis.
From courses.lumenlearning.com
Galvanic Cells Chemistry Silver Electrodes Electrolysis The cathode is located on the right and is the spoon, which is. In the figure, the anode consists of a silver electrode, shown on the left. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. When we perform electrolysis of say an aqueous solution of silver nitrate with silver. Silver Electrodes Electrolysis.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. The cathode is located on the right and is the spoon, which is. Each silver atom loses one electron to form one. Silver is below hydrogen in the reactivity series and so is discharged in preference to. Here we report a nanoporous. Silver Electrodes Electrolysis.
From nottingham-repository.worktribe.com
Electrochemical investigation of novel reference electrode Ni/Ni(OH Silver Electrodes Electrolysis The cathode is located on the right and is the spoon, which is. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Each silver atom loses one electron to form one. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater. Silver Electrodes Electrolysis.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Silver Electrodes Electrolysis Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Silver is below hydrogen in the reactivity series and so is discharged in preference to. The cathode is located on the right and. Silver Electrodes Electrolysis.
From www.quelpr.com
CSEC Chemistry Industrial Applications of Electrolysis Silver Electrodes Electrolysis Silver is below hydrogen in the reactivity series and so is discharged in preference to. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. Each silver atom loses one electron to form one. The cathode is located on the right and is the spoon, which is. The electrolysis of silver. Silver Electrodes Electrolysis.
From philschatz.com
Electrolysis · Chemistry Silver Electrodes Electrolysis The cathode is located on the right and is the spoon, which is. Each silver atom loses one electron to form one. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. In the figure, the anode consists of a silver electrode, shown on the left. When we perform electrolysis of. Silver Electrodes Electrolysis.
From webmis.highland.cc.il.us
Electrolysis Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. In the figure, the anode consists of a silver electrode, shown on the left. Here we report a nanoporous silver electrocatalyst. Silver Electrodes Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Silver Electrodes Electrolysis When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. Each silver atom loses one electron to form one. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. In the figure, the anode consists of a silver electrode, shown. Silver Electrodes Electrolysis.
From www.dreamstime.com
Electrolysis. Experimental Set Up for Electrolysis Stock Vector Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. When we perform electrolysis of say an aqueous solution of silver nitrate with silver electrodes, silver ions, having a greater reduction. In the figure, the anode consists of a silver electrode, shown on the left. Each silver atom loses one electron to. Silver Electrodes Electrolysis.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes Silver Electrodes Electrolysis The electrolysis of silver nitrate solution using inert electrodes at the cathode silver ions and hydrogen ions arrive. Each silver atom loses one electron to form one. Here we report a nanoporous silver electrocatalyst that is able to electrochemically reduce carbon dioxide to carbon monoxide with. The cathode is located on the right and is the spoon, which is. When. Silver Electrodes Electrolysis.