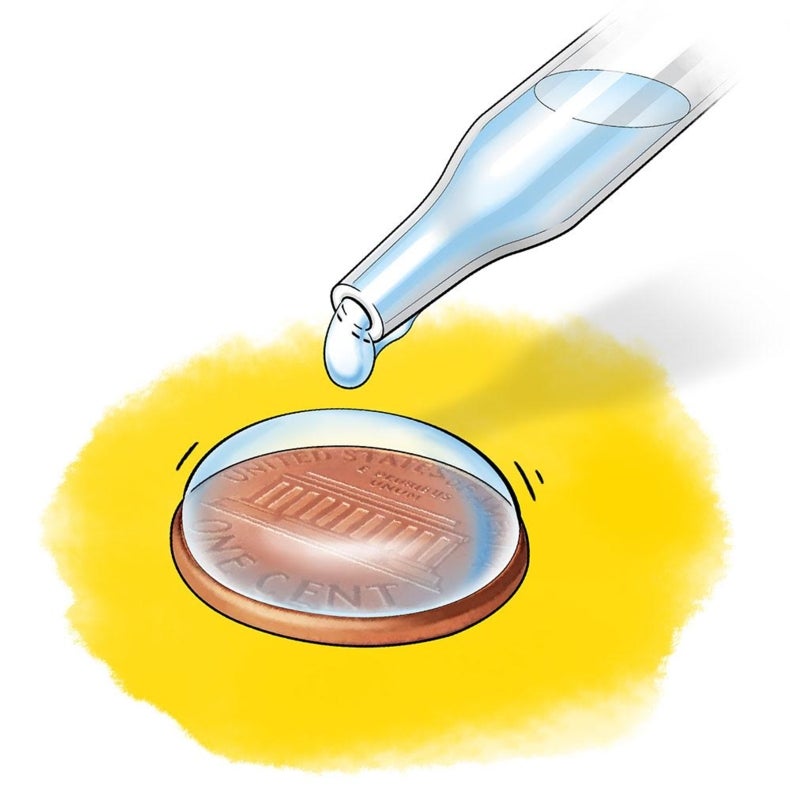
Surface Tension Of Water On Penny . In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. Once the water has reached the edge, you begin to see a bubble. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Fill a glass, cup, or small bowl with tap water. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. Fill the medicine dropper with water. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the.
from www.scientificamerican.com
Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. Fill a glass, cup, or small bowl with tap water. Once the water has reached the edge, you begin to see a bubble. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. Fill the medicine dropper with water. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge.
Measure Surface Tension with a Penny Scientific American
Surface Tension Of Water On Penny Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. Once the water has reached the edge, you begin to see a bubble. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. Fill the medicine dropper with water. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. Fill a glass, cup, or small bowl with tap water.
From frugalfun4boys.com
Drops of Water on a Penny {Awesome Science!} Frugal Fun For Boys and Surface Tension Of Water On Penny The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. They will compare the way water behaves with the less polar liquid isopropyl alcohol. Surface Tension Of Water On Penny.
From www.flickr.com
Drops on a Penny Macro Demonstration of surface tension at… Flickr Surface Tension Of Water On Penny Once the water has reached the edge, you begin to see a bubble. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. In this experiment, you will prove that soap. Surface Tension Of Water On Penny.
From www.youtube.com
Surface Tension Super Ability Of Liquid Water on Penny Experiment Surface Tension Of Water On Penny Fill the medicine dropper with water. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. Once the water has reached the edge, you begin to see a bubble. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. They will compare the. Surface Tension Of Water On Penny.
From www.sciencebuddies.org
Measuring Surface Tension of Water with a Penny Science Project Surface Tension Of Water On Penny Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. In this activity,. Surface Tension Of Water On Penny.
From ar.inspiredpencil.com
Surface Tension Of Water On A Penny Surface Tension Of Water On Penny Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. Fill a glass, cup, or small bowl with tap water. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top. Surface Tension Of Water On Penny.
From 9gag.com
Surface tension of water on a penny 9GAG Surface Tension Of Water On Penny In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. In this activity you will see how soap decreases the surface tension of water. Surface Tension Of Water On Penny.
From ar.inspiredpencil.com
Surface Tension Of Water On A Penny Surface Tension Of Water On Penny In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. Once the water has reached the edge, you begin to see a bubble. Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. The cohesion and surface tension of. Surface Tension Of Water On Penny.
From www.sciencebuddies.org
Measuring Surface Tension of Water with a Penny Surface Tension Of Water On Penny In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the. Surface Tension Of Water On Penny.
From www.shutterstock.com
Demonstration Surface Tension On Penny Using Stock Photo 107199041 Surface Tension Of Water On Penny Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you. Surface Tension Of Water On Penny.
From studylib.net
Penny Lab and Surface Tension Surface Tension Of Water On Penny Once the water has reached the edge, you begin to see a bubble. Fill a glass, cup, or small bowl with tap water. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the.. Surface Tension Of Water On Penny.
From za.pinterest.com
What is surface tension? See how many drops of water you can put on a Surface Tension Of Water On Penny They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on. Surface Tension Of Water On Penny.
From frugalfun4boys.com
Drops of Water on a Penny {Awesome Science!} Frugal Fun For Boys and Surface Tension Of Water On Penny In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. Fill a glass, cup, or small bowl with tap water. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. Middleschoolscience.com) in this portion of the lab you will determine which. Surface Tension Of Water On Penny.
From frugalfun4boys.com
Drops of Water on a Penny {Awesome Science!} Frugal Fun For Boys and Surface Tension Of Water On Penny The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. In this activity you will. Surface Tension Of Water On Penny.
From thesciencekiddo.blogspot.com
Surface Tension Drops of Water on a Coin + Free Printable The Surface Tension Of Water On Penny Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. Fill a glass, cup, or small bowl with tap water. Once the water has reached the edge, you begin to see a bubble. In this experiment, you will. Surface Tension Of Water On Penny.
From www.reddit.com
Surface Tension with a Penny. r/gifs Surface Tension Of Water On Penny Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Fill a glass, cup, or small bowl with tap water. In this activity you will see how soap decreases the surface tension of. Surface Tension Of Water On Penny.
From agrilifelearn.tamu.edu
Water Drops on a Penny Publications AgriLife Learn Surface Tension Of Water On Penny In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. In this activity, you’ll experience surface tension and cohesion by testing how many drops. Surface Tension Of Water On Penny.
From www.sciencekiddo.com
Coin and Water Experiment Water Drops on a Penny • The Science Kiddo Surface Tension Of Water On Penny In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. In this activity, you’ll experience surface tension and cohesion by testing how many drops. Surface Tension Of Water On Penny.
From www.scientificamerican.com
Measure Surface Tension with a Penny Scientific American Surface Tension Of Water On Penny In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Place your penny on a flat, level surface that can get a little wet,. Surface Tension Of Water On Penny.
From www.youtube.com
Surface Tension on a Penny YouTube Surface Tension Of Water On Penny Fill the medicine dropper with water. Fill a glass, cup, or small bowl with tap water. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. In this activity, you’ll experience surface tension. Surface Tension Of Water On Penny.
From easyscienceforkids.com
Comparing Surface Tension of Liquids with Pennies Physics Experiment Surface Tension Of Water On Penny The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. Fill the medicine dropper with water. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Once the water has reached the edge, you. Surface Tension Of Water On Penny.
From www.pinterest.co.uk
Surface Tension Water Experiment Water Drops On A Penny Science Surface Tension Of Water On Penny Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny.. Surface Tension Of Water On Penny.
From thesciencekiddo.blogspot.com
Surface Tension Drops of Water on a Coin + Free Printable The Surface Tension Of Water On Penny In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny.. Surface Tension Of Water On Penny.
From www.youtube.com
Water Surface Tension Coin Trick Home Physics Experiment YouTube Surface Tension Of Water On Penny Fill the medicine dropper with water. Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Middleschoolscience.com) in this portion of the lab you will determine which liquid has. Surface Tension Of Water On Penny.
From www.youtube.com
Surface Tension of Water on a Penny YouTube Surface Tension Of Water On Penny In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Fill a glass, cup, or small bowl with tap water. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. Fill the medicine dropper. Surface Tension Of Water On Penny.
From www.youtube.com
Surface Tension Of Water On A Penny YouTube Surface Tension Of Water On Penny The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. Place your penny on a flat, level surface that can get a little wet,. Surface Tension Of Water On Penny.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension Of Water On Penny In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Once the water has reached the edge, you begin to see a bubble. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. Fill. Surface Tension Of Water On Penny.
From www.pinterest.com
Surface Tension Water Experiment Water drops on a penny science Surface Tension Of Water On Penny Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. In this experiment, you will prove that soap decreases the surface tension. Surface Tension Of Water On Penny.
From www.youtube.com
Water surface tension experiment (how many drops will fit on the penny Surface Tension Of Water On Penny Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. Fill a glass, cup, or small bowl with tap water. Middleschoolscience.com) in this portion of the lab you will determine which liquid has the. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. In this. Surface Tension Of Water On Penny.
From sciencekiddo.com
Coin and Water Experiment Water Drops on a Penny • The Science Kiddo Surface Tension Of Water On Penny In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. The cohesion and surface tension of water becomes apparent when the drops of water. Surface Tension Of Water On Penny.
From www.flickr.com
Water on a penny lf411 Flickr Surface Tension Of Water On Penny In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. Fill a glass, cup, or small bowl with tap water. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. Middleschoolscience.com) in this portion of the lab you will determine which. Surface Tension Of Water On Penny.
From mombrite.ck.page
Free Drops of Water on a Penny Worksheet Surface Tension Of Water On Penny Fill a glass, cup, or small bowl with tap water. The cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. In this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. Place your penny on. Surface Tension Of Water On Penny.
From www.flowvis.org
Surface tension holds water on a penny, taller than the penny itself Surface Tension Of Water On Penny They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. Fill a glass, cup, or small bowl with tap water. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. The cohesion and surface tension of water becomes apparent when the. Surface Tension Of Water On Penny.
From www.youtube.com
Surface tension of water on a penny YouTube Surface Tension Of Water On Penny Place your penny on a flat, level surface that can get a little wet, like a kitchen counter. They will compare the way water behaves with the less polar liquid isopropyl alcohol and will. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. Middleschoolscience.com) in this. Surface Tension Of Water On Penny.
From deceptivelyeducational.blogspot.com.au
Relentlessly Fun, Deceptively Educational How many drops of water fit Surface Tension Of Water On Penny Fill a glass, cup, or small bowl with tap water. Once the water has reached the edge, you begin to see a bubble. In this activity, you’ll experience surface tension and cohesion by testing how many drops of water you can you fit on a penny. They will compare the way water behaves with the less polar liquid isopropyl alcohol. Surface Tension Of Water On Penny.
From www.rookieparenting.com
Surface Tension Water Experiment Water Drops On A Penny Surface Tension Of Water On Penny Once the water has reached the edge, you begin to see a bubble. In this experiment, you will prove that soap decreases the surface tension of water by putting water droplets on top of a penny. Fill a glass, cup, or small bowl with tap water. The cohesion and surface tension of water becomes apparent when the drops of water. Surface Tension Of Water On Penny.