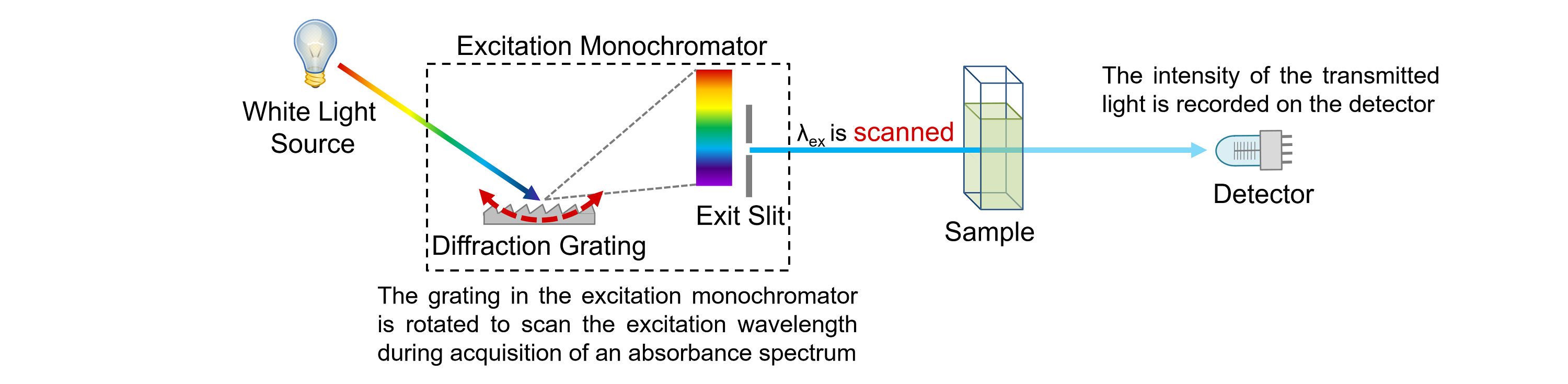
What Does Absorbance Mean In Spectrophotometry . Measuring the absorbance of a solution. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. It is also known as optical. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. For a = 1, 10% of the light is. absorption occurs when the energy of the light matches the energy needed to move electrons within the. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. as light passes through a sample, its power decreases as some of it is absorbed. Transmittance is the quantity of light that. If you have read the page about how an absorption spectrometer works, you will know that it passes a. This attenuation of radiation is described quantitatively. absorbance is a measure of the quantity of light absorbed by a sample.
from shaniyaukung.blogspot.com
Transmittance is the quantity of light that. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. It is also known as optical. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. absorbance is a measure of the quantity of light absorbed by a sample. This attenuation of radiation is described quantitatively. Measuring the absorbance of a solution. absorption occurs when the energy of the light matches the energy needed to move electrons within the. as light passes through a sample, its power decreases as some of it is absorbed.
What Is Absorbance In Spectrophotometer
What Does Absorbance Mean In Spectrophotometry Measuring the absorbance of a solution. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. Transmittance is the quantity of light that. Measuring the absorbance of a solution. For a = 1, 10% of the light is. This attenuation of radiation is described quantitatively. absorbance is a measure of the quantity of light absorbed by a sample. as light passes through a sample, its power decreases as some of it is absorbed. It is also known as optical. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. If you have read the page about how an absorption spectrometer works, you will know that it passes a. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. absorption occurs when the energy of the light matches the energy needed to move electrons within the. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution.
From namrataheda.blogspot.com
B for Biology Spectrophotometry Atomic Absorption Spectrophotometry What Does Absorbance Mean In Spectrophotometry the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. as light passes through a sample, its power decreases as some of it is absorbed. If you have read the page about how an absorption spectrometer works, you will know that it passes a. It is also known as. What Does Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Fundamentals of Spectrophotometry PowerPoint Presentation, free What Does Absorbance Mean In Spectrophotometry Transmittance is the quantity of light that. absorption occurs when the energy of the light matches the energy needed to move electrons within the. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. as light passes through a sample, its power decreases as some of it is absorbed. the. What Does Absorbance Mean In Spectrophotometry.
From chem.libretexts.org
Spectrophotometry Chemistry LibreTexts What Does Absorbance Mean In Spectrophotometry each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. absorption occurs when the energy of the light matches the energy needed to move electrons within the. the absorbance (a) is defined as the log. What Does Absorbance Mean In Spectrophotometry.
From gioxxitjp.blob.core.windows.net
What Is Uv Absorbance at Scott Francis blog What Does Absorbance Mean In Spectrophotometry It is also known as optical. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best.. What Does Absorbance Mean In Spectrophotometry.
From www.researchgate.net
The relationship between absorbance measured on a spectrophotometer What Does Absorbance Mean In Spectrophotometry as light passes through a sample, its power decreases as some of it is absorbed. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. Transmittance is the quantity of light. What Does Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does Absorbance Mean In Spectrophotometry the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. For a = 1, 10% of the light is. Transmittance is the quantity of light that. It is also known as optical. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. . What Does Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does Absorbance Mean In Spectrophotometry absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. absorbance is a measure of the quantity of light absorbed by a sample. It is also known as optical. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. each. What Does Absorbance Mean In Spectrophotometry.
From www.scribd.com
Spectrophotometry PDF Spectrophotometry Absorbance What Does Absorbance Mean In Spectrophotometry If you have read the page about how an absorption spectrometer works, you will know that it passes a. For a = 1, 10% of the light is. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. you need a spectrometer to produce a variety of wavelengths because different compounds. What Does Absorbance Mean In Spectrophotometry.
From www.youtube.com
Identifying Chemicals with Spectrophotometry YouTube What Does Absorbance Mean In Spectrophotometry as light passes through a sample, its power decreases as some of it is absorbed. absorbance is a measure of the quantity of light absorbed by a sample. For a = 1, 10% of the light is. Measuring the absorbance of a solution. If you have read the page about how an absorption spectrometer works, you will know. What Does Absorbance Mean In Spectrophotometry.
From theamberpost.com
Understanding Absorbance Reading Shedding Light on Quantitative What Does Absorbance Mean In Spectrophotometry the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. This attenuation of radiation is described quantitatively. Measuring the absorbance of a solution. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. It is also known as optical. If you have. What Does Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Fundamentals of Spectrophotometry PowerPoint Presentation, free What Does Absorbance Mean In Spectrophotometry If you have read the page about how an absorption spectrometer works, you will know that it passes a. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. Measuring the absorbance of a solution. It is also known as optical. you need a spectrometer to produce a variety of wavelengths. What Does Absorbance Mean In Spectrophotometry.
From giorbeiku.blob.core.windows.net
Absorbance Units at Annie Haley blog What Does Absorbance Mean In Spectrophotometry Measuring the absorbance of a solution. For a = 1, 10% of the light is. It is also known as optical. absorbance is a measure of the quantity of light absorbed by a sample. If you have read the page about how an absorption spectrometer works, you will know that it passes a. absorbance (a), also known as. What Does Absorbance Mean In Spectrophotometry.
From fyopbfszs.blob.core.windows.net
Difference Between Turbidity And Absorbance at William Harville blog What Does Absorbance Mean In Spectrophotometry each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. This attenuation of radiation is described quantitatively. absorbance is a measure of the quantity of light absorbed by a sample. absorbance. What Does Absorbance Mean In Spectrophotometry.
From exyoefqjv.blob.core.windows.net
Complementary Colors In Spectrophotometry at Ignacio Ulrich blog What Does Absorbance Mean In Spectrophotometry each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. This attenuation of radiation is described quantitatively. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. If you have read the page about how an absorption spectrometer works, you will know that it passes. What Does Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry Basics PowerPoint Presentation, free download What Does Absorbance Mean In Spectrophotometry If you have read the page about how an absorption spectrometer works, you will know that it passes a. as light passes through a sample, its power decreases as some of it is absorbed. absorbance is a measure of the quantity of light absorbed by a sample. Transmittance is the quantity of light that. absorbance (a), also. What Does Absorbance Mean In Spectrophotometry.
From www.nist.gov
Spectrophotometry NIST What Does Absorbance Mean In Spectrophotometry Measuring the absorbance of a solution. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. absorbance is a measure of the quantity of light absorbed by a sample. absorption occurs when the energy of the light matches the energy needed to move electrons within the. For a. What Does Absorbance Mean In Spectrophotometry.
From www.scribd.com
SPECTROPHOTOMETRY PDF Spectrophotometry Absorbance What Does Absorbance Mean In Spectrophotometry as light passes through a sample, its power decreases as some of it is absorbed. Measuring the absorbance of a solution. For a = 1, 10% of the light is. This attenuation of radiation is described quantitatively. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. each unit in absorbance corresponds. What Does Absorbance Mean In Spectrophotometry.
From www.researchgate.net
Multiple peaks in UVVIS absorbance spectra, What do the mean What Does Absorbance Mean In Spectrophotometry absorbance is a measure of the quantity of light absorbed by a sample. It is also known as optical. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. For a = 1, 10% of the light is. Transmittance is the quantity of light that. as light passes through a sample,. What Does Absorbance Mean In Spectrophotometry.
From www.vrogue.co
What Units Are Used To Measure Absorbance On A Spectr vrogue.co What Does Absorbance Mean In Spectrophotometry If you have read the page about how an absorption spectrometer works, you will know that it passes a. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. as light passes through a sample, its power decreases as some of it is absorbed. Transmittance is the quantity of. What Does Absorbance Mean In Spectrophotometry.
From shaniyaukung.blogspot.com
What Is Absorbance In Spectrophotometer What Does Absorbance Mean In Spectrophotometry For a = 1, 10% of the light is. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. If you have read the page about how an absorption spectrometer works, you will know that. What Does Absorbance Mean In Spectrophotometry.
From www.scribd.com
Spectrophotometry 1 PDF Spectrophotometry Absorbance What Does Absorbance Mean In Spectrophotometry absorption occurs when the energy of the light matches the energy needed to move electrons within the. absorbance is a measure of the quantity of light absorbed by a sample. If you have read the page about how an absorption spectrometer works, you will know that it passes a. each unit in absorbance corresponds with an order. What Does Absorbance Mean In Spectrophotometry.
From einvoice.fpt.com.vn
SOLVED Based On The Graph Of Absorbance Versus Wavelength, 58 OFF What Does Absorbance Mean In Spectrophotometry It is also known as optical. For a = 1, 10% of the light is. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. as light passes through a sample, its power decreases. What Does Absorbance Mean In Spectrophotometry.
From www.chemistrystudent.com
IR (Infrared Spectroscopy) (ALevel) ChemistryStudent What Does Absorbance Mean In Spectrophotometry Measuring the absorbance of a solution. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. It is also known as optical. If you have read the page about how an absorption spectrometer works, you will know that it passes a. This attenuation of radiation is described quantitatively. absorbance (a), also known as. What Does Absorbance Mean In Spectrophotometry.
From sciencenotes.org
Beer's Law Equation and Example What Does Absorbance Mean In Spectrophotometry For a = 1, 10% of the light is. absorption occurs when the energy of the light matches the energy needed to move electrons within the. Transmittance is the quantity of light that. as light passes through a sample, its power decreases as some of it is absorbed. This attenuation of radiation is described quantitatively. each unit. What Does Absorbance Mean In Spectrophotometry.
From namrataheda.blogspot.com
B for Biology Spectrophotometry UVVisible Spectrophotometry What Does Absorbance Mean In Spectrophotometry absorbance is a measure of the quantity of light absorbed by a sample. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. Measuring the absorbance of a solution. you. What Does Absorbance Mean In Spectrophotometry.
From www.vernier.com
Decoding Your Absorbance Readings Vernier What Does Absorbance Mean In Spectrophotometry It is also known as optical. If you have read the page about how an absorption spectrometer works, you will know that it passes a. as light passes through a sample, its power decreases as some of it is absorbed. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. For a. What Does Absorbance Mean In Spectrophotometry.
From gionlujil.blob.core.windows.net
Does Spectrophotometer Calculate Absorbance at Ronald Gonzalez blog What Does Absorbance Mean In Spectrophotometry It is also known as optical. If you have read the page about how an absorption spectrometer works, you will know that it passes a. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. Measuring the absorbance of a solution. This attenuation of radiation is described quantitatively. each unit in absorbance corresponds. What Does Absorbance Mean In Spectrophotometry.
From streetlink.org.uk
⭐ What is a spectrophotometer used for in biology. Spectrophotometer What Does Absorbance Mean In Spectrophotometry absorption occurs when the energy of the light matches the energy needed to move electrons within the. If you have read the page about how an absorption spectrometer works, you will know that it passes a. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. as light passes through. What Does Absorbance Mean In Spectrophotometry.
From www.slideshare.net
Spectrophotometry What Does Absorbance Mean In Spectrophotometry If you have read the page about how an absorption spectrometer works, you will know that it passes a. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. absorption occurs when the energy of the light matches the energy needed to move electrons within the. For a = 1, 10%. What Does Absorbance Mean In Spectrophotometry.
From definitionhjo.blogspot.com
Definition Of Absorbance In Chemistry DEFINITION HJO What Does Absorbance Mean In Spectrophotometry This attenuation of radiation is described quantitatively. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. If you have read the page about how an absorption spectrometer works, you will know that it passes a.. What Does Absorbance Mean In Spectrophotometry.
From exyvsvafc.blob.core.windows.net
Define Spectrophotometry In Chemistry at Amanda Isom blog What Does Absorbance Mean In Spectrophotometry This attenuation of radiation is described quantitatively. If you have read the page about how an absorption spectrometer works, you will know that it passes a. Transmittance is the quantity of light that. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. For a = 1, 10% of the light is.. What Does Absorbance Mean In Spectrophotometry.
From ibsen.com
Absorption spectroscopy Ibsen Photonics What Does Absorbance Mean In Spectrophotometry as light passes through a sample, its power decreases as some of it is absorbed. For a = 1, 10% of the light is. absorbance (a), also known as optical density (od), is the quantity of light absorbed by a solution. the absorbance (a) is defined as the log of the ratio of the light intensity entering. What Does Absorbance Mean In Spectrophotometry.
From exyoefqjv.blob.core.windows.net
Complementary Colors In Spectrophotometry at Ignacio Ulrich blog What Does Absorbance Mean In Spectrophotometry Transmittance is the quantity of light that. It is also known as optical. the absorbance (a) is defined as the log of the ratio of the light intensity entering to that exiting. each unit in absorbance corresponds with an order of magnitude in the fraction of light transmitted. For a = 1, 10% of the light is. . What Does Absorbance Mean In Spectrophotometry.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID6911139 What Does Absorbance Mean In Spectrophotometry This attenuation of radiation is described quantitatively. Transmittance is the quantity of light that. absorbance is a measure of the quantity of light absorbed by a sample. It is also known as optical. you need a spectrometer to produce a variety of wavelengths because different compounds absorb best. absorption occurs when the energy of the light matches. What Does Absorbance Mean In Spectrophotometry.
From scienceinfo.com
Atomic Absorption Spectrophotometry Principle, Parts, Uses What Does Absorbance Mean In Spectrophotometry as light passes through a sample, its power decreases as some of it is absorbed. For a = 1, 10% of the light is. absorbance is a measure of the quantity of light absorbed by a sample. Measuring the absorbance of a solution. each unit in absorbance corresponds with an order of magnitude in the fraction of. What Does Absorbance Mean In Spectrophotometry.