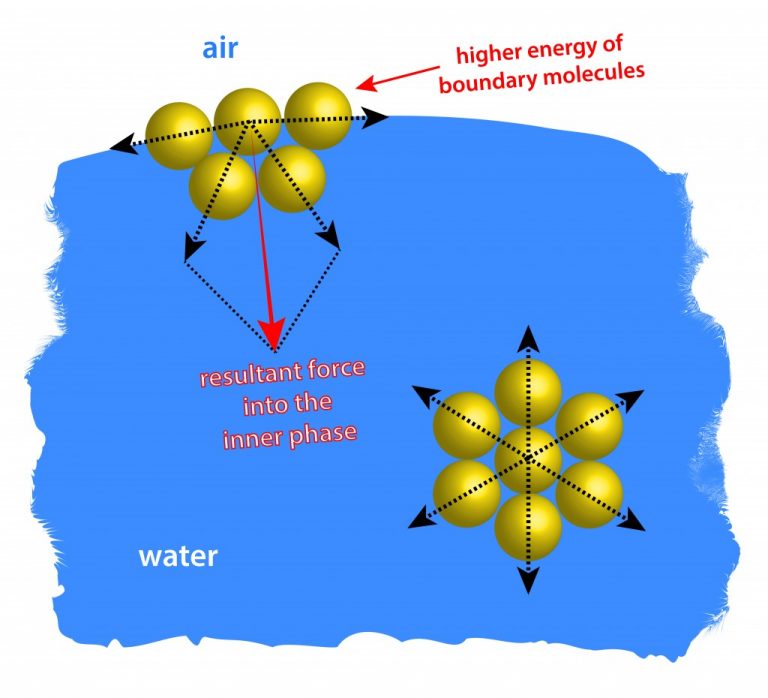
How Does Surface Tension Relate To Hydrogen Bonding . This drawing highlights two h This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Surface tension refers to how strongly molecules are held to the surface of a liquid. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; These molecules pull downwards on the surface molecules causing the. These molecules pull downwards the. As a result of hydrogen bonds which exert a strong downwards force on water molecules at. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. Same goes for other liquids, even hydrophobic liquids such as oil. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Hydrogen bonds contribute to surface tension by creating a strong, interconnected network of water molecules at the surface. Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to.
from www.scienceabc.com
As a result of hydrogen bonds which exert a strong downwards force on water molecules at. Same goes for other liquids, even hydrophobic liquids such as oil. Hydrogen bonds contribute to surface tension by creating a strong, interconnected network of water molecules at the surface. These molecules pull downwards on the surface molecules causing the. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Surface tension refers to how strongly molecules are held to the surface of a liquid.
Surface Tension Definition, Explanation, Examples And Significance
How Does Surface Tension Relate To Hydrogen Bonding This drawing highlights two h There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Surface tension is a manifestation of the presence of the hydrogen bond. These molecules pull downwards on the surface molecules causing the. These molecules pull downwards the. Surface tension refers to how strongly molecules are held to the surface of a liquid. As a result of hydrogen bonds which exert a strong downwards force on water molecules at. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes for other liquids, even hydrophobic liquids such as oil. This drawing highlights two h The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. Hydrogen bonds contribute to surface tension by creating a strong, interconnected network of water molecules at the surface. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Those molecules of water that are at the surface are strongly attracted to. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds;
From errantscience.com
Water surface tension ErrantScience How Does Surface Tension Relate To Hydrogen Bonding Surface tension is a manifestation of the presence of the hydrogen bond. The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. This drawing highlights two h Those molecules of water that are at the surface are strongly attracted to. The water molecules at the. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideshare.net
Water, Hydrogen Bonds, and the Hydrologic Cycle How Does Surface Tension Relate To Hydrogen Bonding Those molecules of water that are at the surface are strongly attracted to. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The difference between the forces experienced by a molecule at the. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Chemical Properties of Atoms PowerPoint Presentation, free How Does Surface Tension Relate To Hydrogen Bonding The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes for other liquids, even hydrophobic liquids such as oil. Those molecules of water that are at the surface are strongly attracted to. Surface tension refers to how strongly molecules are held to the surface of a liquid. Hydrogen bonds contribute to. How Does Surface Tension Relate To Hydrogen Bonding.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Relate To Hydrogen Bonding Same goes for other liquids, even hydrophobic liquids such as oil. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. This drawing highlights two h The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Surface tension refers to. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Relate To Hydrogen Bonding Surface tension is a manifestation of the presence of the hydrogen bond. Surface tension refers to how strongly molecules are held to the surface of a liquid. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The difference between the forces experienced by a molecule at. How Does Surface Tension Relate To Hydrogen Bonding.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion How Does Surface Tension Relate To Hydrogen Bonding There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; This drawing highlights two h Surface tension is a manifestation of the presence of the hydrogen bond. These molecules pull. How Does Surface Tension Relate To Hydrogen Bonding.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion How Does Surface Tension Relate To Hydrogen Bonding This drawing highlights two h The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to. How Does Surface Tension Relate To Hydrogen Bonding.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Does Surface Tension Relate To Hydrogen Bonding Surface tension is a manifestation of the presence of the hydrogen bond. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. Same goes for other. How Does Surface Tension Relate To Hydrogen Bonding.
From pediaa.com
Difference Between Intermolecular and Intramolecular Hydrogen Bonding How Does Surface Tension Relate To Hydrogen Bonding This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Those molecules of water that are at the surface are strongly attracted to. Same goes for other liquids, even hydrophobic liquids such as oil. This drawing highlights two h There are forces between the liquid such as van der waals forces. How Does Surface Tension Relate To Hydrogen Bonding.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Relate To Hydrogen Bonding The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; This drawing highlights two h These molecules pull downwards on the surface molecules causing the. Surface tension refers to how strongly molecules are held to the surface of a liquid. As a result of hydrogen bonds which exert a strong downwards force on. How Does Surface Tension Relate To Hydrogen Bonding.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Relate To Hydrogen Bonding The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise. How Does Surface Tension Relate To Hydrogen Bonding.
From universe-review.ca
Molecules How Does Surface Tension Relate To Hydrogen Bonding Those molecules of water that are at the surface are strongly attracted to. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. As a result of hydrogen bonds which exert a strong downwards force on water molecules at. Surface tension is a manifestation of the presence of the hydrogen bond.. How Does Surface Tension Relate To Hydrogen Bonding.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion How Does Surface Tension Relate To Hydrogen Bonding This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. These molecules pull downwards the. Surface tension refers to how strongly molecules are held to the surface of a liquid. As a result of hydrogen bonds which exert a strong downwards force on water molecules at. The water molecules at the. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Hydrogen Bonding PowerPoint Presentation, free download ID297441 How Does Surface Tension Relate To Hydrogen Bonding Surface tension refers to how strongly molecules are held to the surface of a liquid. These molecules pull downwards the. As a result of hydrogen bonds which exert a strong downwards force on water molecules at. This drawing highlights two h The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Biology Biochemistry Unit Chapter 2 The Chemistry of Life How Does Surface Tension Relate To Hydrogen Bonding There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; As a result of hydrogen bonds which exert a strong downwards force on water molecules at. Surface tension is a. How Does Surface Tension Relate To Hydrogen Bonding.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Relate To Hydrogen Bonding This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Hydrogen bonds contribute to surface tension by creating a strong, interconnected network of water molecules at the surface. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Surface tension refers to how strongly. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint How Does Surface Tension Relate To Hydrogen Bonding This drawing highlights two h These molecules pull downwards on the surface molecules causing the. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Surface tension refers to how strongly molecules are held to the surface of a liquid. As a result of hydrogen bonds which exert a strong downwards. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Chapter 15 “Water and Aqueous Systems” PowerPoint Presentation How Does Surface Tension Relate To Hydrogen Bonding This drawing highlights two h Those molecules of water that are at the surface are strongly attracted to. These molecules pull downwards the. As a result of hydrogen bonds which exert a strong downwards force on water molecules at. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within. How Does Surface Tension Relate To Hydrogen Bonding.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Does Surface Tension Relate To Hydrogen Bonding As a result of hydrogen bonds which exert a strong downwards force on water molecules at. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; These molecules pull downwards on the surface molecules causing the. There are forces between the liquid such as van der waals forces that are responsible for the. How Does Surface Tension Relate To Hydrogen Bonding.
From www.savemyexams.com
Hydrogen Bonding Edexcel A Level Chemistry Revision Notes 2017 How Does Surface Tension Relate To Hydrogen Bonding Surface tension is a manifestation of the presence of the hydrogen bond. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes for other liquids, even hydrophobic liquids such as oil. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The. How Does Surface Tension Relate To Hydrogen Bonding.
From lessonschoolsimplistic.z5.web.core.windows.net
How To Explain Surface Tension To Kids How Does Surface Tension Relate To Hydrogen Bonding These molecules pull downwards the. Those molecules of water that are at the surface are strongly attracted to. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Surface tension refers to how strongly molecules are held to the surface of a liquid. This drawing highlights two. How Does Surface Tension Relate To Hydrogen Bonding.
From www.chemedx.org
Measuring Surface Tension to Investigate Intermolecular Forces How Does Surface Tension Relate To Hydrogen Bonding Surface tension refers to how strongly molecules are held to the surface of a liquid. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Those molecules of water that are at the surface are strongly attracted to. Same goes for other liquids, even hydrophobic liquids such. How Does Surface Tension Relate To Hydrogen Bonding.
From mungfali.com
Hydrogen Bonding Forces How Does Surface Tension Relate To Hydrogen Bonding This drawing highlights two h The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Those molecules of water that are at the surface are strongly attracted to. Surface tension is a manifestation of the presence of the hydrogen bond. Hydrogen bonds contribute to surface tension by creating a strong, interconnected network of. How Does Surface Tension Relate To Hydrogen Bonding.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Relate To Hydrogen Bonding The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes for other liquids, even hydrophobic liquids such as oil. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; The difference between the forces experienced by a molecule at the surface and one in. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID How Does Surface Tension Relate To Hydrogen Bonding The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen. How Does Surface Tension Relate To Hydrogen Bonding.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Does Surface Tension Relate To Hydrogen Bonding The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes for other liquids, even hydrophobic liquids such as oil. This drawing highlights two h The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. Surface tension. How Does Surface Tension Relate To Hydrogen Bonding.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion How Does Surface Tension Relate To Hydrogen Bonding Surface tension is a manifestation of the presence of the hydrogen bond. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. These molecules pull downwards the. Those molecules of water that are at the surface are strongly attracted to. There are forces between the liquid such as van der waals. How Does Surface Tension Relate To Hydrogen Bonding.
From slidetodoc.com
POLARITY INTERMOLECULAR FORCES HYBRIDIZATION Molecular Polarity The uneven How Does Surface Tension Relate To Hydrogen Bonding This drawing highlights two h The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes for other liquids, even hydrophobic liquids such as oil. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Hydrogen bonds contribute to surface tension by creating. How Does Surface Tension Relate To Hydrogen Bonding.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion How Does Surface Tension Relate To Hydrogen Bonding Those molecules of water that are at the surface are strongly attracted to. Surface tension refers to how strongly molecules are held to the surface of a liquid. These molecules pull downwards the. This drawing highlights two h The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; The difference between the forces. How Does Surface Tension Relate To Hydrogen Bonding.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii How Does Surface Tension Relate To Hydrogen Bonding The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. As a result of hydrogen bonds which exert a strong downwards force on water molecules at. Same goes for other liquids, even hydrophobic liquids such as oil. This drawing highlights two h This is why. How Does Surface Tension Relate To Hydrogen Bonding.
From bio1100.nicerweb.com
surface_tension/02_13 How Does Surface Tension Relate To Hydrogen Bonding The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. These molecules pull downwards the. Those molecules of water that are at the surface are strongly attracted to. Surface tension is a manifestation of the presence of the hydrogen bond. Surface tension refers to how. How Does Surface Tension Relate To Hydrogen Bonding.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Relate To Hydrogen Bonding These molecules pull downwards on the surface molecules causing the. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Same goes for other liquids, even hydrophobic liquids such as oil. The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; As a result of hydrogen. How Does Surface Tension Relate To Hydrogen Bonding.
From www.pinterest.com
Surface tension, Surface, Tension How Does Surface Tension Relate To Hydrogen Bonding Surface tension is a manifestation of the presence of the hydrogen bond. Surface tension refers to how strongly molecules are held to the surface of a liquid. The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension. These molecules pull downwards on the surface molecules. How Does Surface Tension Relate To Hydrogen Bonding.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru How Does Surface Tension Relate To Hydrogen Bonding The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds; Surface tension is a manifestation of the presence of the hydrogen bond. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. As a result of hydrogen bonds which exert. How Does Surface Tension Relate To Hydrogen Bonding.
From www.shutterstock.com
Hydrogen Bonds Surface Tension 库存矢量图(免版税)1640032501 How Does Surface Tension Relate To Hydrogen Bonding Surface tension refers to how strongly molecules are held to the surface of a liquid. Those molecules of water that are at the surface are strongly attracted to. Surface tension is a manifestation of the presence of the hydrogen bond. This drawing highlights two h The difference between the forces experienced by a molecule at the surface and one in. How Does Surface Tension Relate To Hydrogen Bonding.