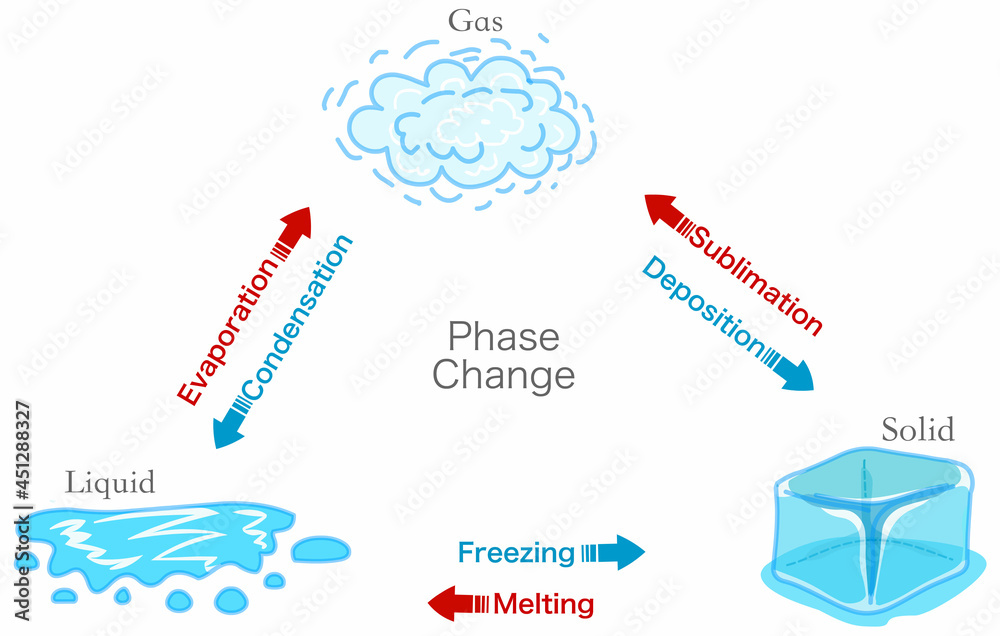
Explain The Process Of Phase Change Of Water . water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. for water, there is no liquid phase at pressures below 0.00600 atm. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. steps of the water cycle: The phase change from solid to gas is called. phase diagram for water. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. One of these special properties is the fact that solid. Water is a unique substance in many ways. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic.
from circuitgonelladrianxm.z22.web.core.windows.net
Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. for water, there is no liquid phase at pressures below 0.00600 atm. The phase change from solid to gas is called. Water is a unique substance in many ways. steps of the water cycle: phase diagram for water. One of these special properties is the fact that solid.
Show The Phase Change Diagram
Explain The Process Of Phase Change Of Water a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. steps of the water cycle: The phase change from solid to gas is called. Water is a unique substance in many ways. phase diagram for water. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. One of these special properties is the fact that solid. for water, there is no liquid phase at pressures below 0.00600 atm. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter.
From www.metlink.org
The Changing Water Cycle Metlink Weather & Climate Teaching Resources Explain The Process Of Phase Change Of Water a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. steps of the water cycle: phase diagram for water. water has the unusual property that ice. Explain The Process Of Phase Change Of Water.
From fixlibrarywrannorrykk.z22.web.core.windows.net
Explain Water Cycle In Detail With Diagram Explain The Process Of Phase Change Of Water Water is a unique substance in many ways. phase diagram for water. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The phase change from solid to gas is called. for water,. Explain The Process Of Phase Change Of Water.
From www.vectorstock.com
Phase transitions of matter in water Royalty Free Vector Explain The Process Of Phase Change Of Water steps of the water cycle: Water is a unique substance in many ways. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma. Explain The Process Of Phase Change Of Water.
From www.slideserve.com
PPT Phase Changes in Water PowerPoint Presentation, free download ID6843357 Explain The Process Of Phase Change Of Water phase diagram for water. The phase change from solid to gas is called. One of these special properties is the fact that solid. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. a phase change or phase transition. Explain The Process Of Phase Change Of Water.
From www.lessonpaths.com
Changing States Of Water LessonPaths Explain The Process Of Phase Change Of Water phase diagram for water. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. One of these special properties is the fact that solid. these changes of state are essential aspects of our earth’s water cycle as well as. Explain The Process Of Phase Change Of Water.
From byjus.com
Phase Diagram of Water Explanation and Diagrammatic Representation of Phase Diagram of Water Explain The Process Of Phase Change Of Water phase diagram for water. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Water is a unique substance in many ways. The phase change from solid to gas is called. One of these special properties is the fact that solid. these changes of state are essential aspects. Explain The Process Of Phase Change Of Water.
From www.chemistrylearner.com
Phase Diagram of Water (H2O) Explain The Process Of Phase Change Of Water Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. steps of the water cycle: phase diagram for water. for water, there is no liquid phase at pressures below 0.00600 atm. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed. Explain The Process Of Phase Change Of Water.
From www.alamy.com
Water States of matter Phase. Change of State for Water Diagram. Changing the state of matter Explain The Process Of Phase Change Of Water steps of the water cycle: Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. One of these special properties is the fact that solid. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. . Explain The Process Of Phase Change Of Water.
From animalia-life.club
Change Of State Diagram For Water Explain The Process Of Phase Change Of Water these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. phase diagram for water. steps of the water cycle: One of these special properties is the fact that solid. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states. Explain The Process Of Phase Change Of Water.
From circuitdiagramlows.z22.web.core.windows.net
Phase Change Diagram For Water Explain The Process Of Phase Change Of Water Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The phase change from solid to gas is called. Water is a unique substance in many ways. steps of the water cycle: One of these special properties is the fact that solid. for water, there is no liquid phase at pressures below 0.00600 atm.. Explain The Process Of Phase Change Of Water.
From ar.inspiredpencil.com
Change Of State Diagram For Water Explain The Process Of Phase Change Of Water a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. for water, there is no liquid phase at pressures below 0.00600 atm. One of these special properties is the fact that solid. phase diagram. Explain The Process Of Phase Change Of Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Explain The Process Of Phase Change Of Water Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. The phase change from solid to gas is called. these changes of state are essential aspects of our earth’s water cycle as well as many. Explain The Process Of Phase Change Of Water.
From guidemanualcoset.z21.web.core.windows.net
Phase Changes And Phase Diagrams Explain The Process Of Phase Change Of Water water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. The phase change from solid to gas is called. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. One of these special properties is the fact. Explain The Process Of Phase Change Of Water.
From circuitgonelladrianxm.z22.web.core.windows.net
Show The Phase Change Diagram Explain The Process Of Phase Change Of Water One of these special properties is the fact that solid. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. phase diagram for water. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. Water is a unique substance in many ways. a. Explain The Process Of Phase Change Of Water.
From www.thoughtco.com
List of Phase Changes Between States of Matter Explain The Process Of Phase Change Of Water these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. One of these special properties is the fact that solid.. Explain The Process Of Phase Change Of Water.
From learnweather.com
Buoyancy and phase changes in water Explain The Process Of Phase Change Of Water One of these special properties is the fact that solid. phase diagram for water. steps of the water cycle: Water is a unique substance in many ways. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. a. Explain The Process Of Phase Change Of Water.
From www.theschoolrun.com
What are states of matter? TheSchoolRun Explain The Process Of Phase Change Of Water a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. One of these special properties is the fact that solid. . Explain The Process Of Phase Change Of Water.
From wirepartnemertines.z5.web.core.windows.net
Changing States Of Matter Diagram Explain The Process Of Phase Change Of Water a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. One of these special properties is the fact that solid. Water is a unique substance in many ways. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. . Explain The Process Of Phase Change Of Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Explain The Process Of Phase Change Of Water One of these special properties is the fact that solid. steps of the water cycle: these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. Water is a unique substance in many ways. water has the unusual property that ice is less dense than liquid water at. Explain The Process Of Phase Change Of Water.
From www.slideserve.com
PPT Basic Chemistry PowerPoint Presentation ID2465261 Explain The Process Of Phase Change Of Water water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. The phase change from solid to gas is called. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. . Explain The Process Of Phase Change Of Water.
From www.jove.com
Phase Diagrams Carbon Dioxide and Water Phase Diagrams Chemistry JoVE Explain The Process Of Phase Change Of Water a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. One of these special properties is the fact that solid. Water is a unique substance in many ways. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The phase change from solid to gas is. Explain The Process Of Phase Change Of Water.
From scienceline.ucsb.edu
UCSB Science Line Explain The Process Of Phase Change Of Water phase diagram for water. The phase change from solid to gas is called. Water is a unique substance in many ways. steps of the water cycle: water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. for water,. Explain The Process Of Phase Change Of Water.
From vhmsscience.weebly.com
Phase Change Boiling Water Lab VISTA HEIGHTS 8TH GRADE SCIENCE Explain The Process Of Phase Change Of Water phase diagram for water. Water is a unique substance in many ways. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. One of these special properties is the fact that solid. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. steps. Explain The Process Of Phase Change Of Water.
From www.worldatlas.com
The Water Cycle WorldAtlas Explain The Process Of Phase Change Of Water these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. Water is a unique substance in many ways. steps. Explain The Process Of Phase Change Of Water.
From www.esa.int
ESA Phase transitions between ice, water and vapour, and the energy released or absorbed Explain The Process Of Phase Change Of Water phase diagram for water. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. steps of the water cycle: water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase. Explain The Process Of Phase Change Of Water.
From www.slideserve.com
PPT Atmospheric Moisture PowerPoint Presentation, free download ID1598948 Explain The Process Of Phase Change Of Water steps of the water cycle: The phase change from solid to gas is called. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. Water is a unique substance in many ways. phase diagram for water. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and. Explain The Process Of Phase Change Of Water.
From www.slideserve.com
PPT Phase Changes in Water PowerPoint Presentation, free download ID6843357 Explain The Process Of Phase Change Of Water these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. Explain The Process Of Phase Change Of Water.
From www.researchgate.net
2 Water phase change diagram Download Scientific Diagram Explain The Process Of Phase Change Of Water for water, there is no liquid phase at pressures below 0.00600 atm. The phase change from solid to gas is called. steps of the water cycle: phase diagram for water. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the. Explain The Process Of Phase Change Of Water.
From manuallistaeschylus.z14.web.core.windows.net
Phase Change Diagram Of Water Blank Explain The Process Of Phase Change Of Water Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The phase change from solid to gas is called. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes. Explain The Process Of Phase Change Of Water.
From atocwatervapor.blogspot.com
Water Phase Change Sublimation Explain The Process Of Phase Change Of Water water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from. for water, there is no liquid phase at pressures below 0.00600 atm. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states. Explain The Process Of Phase Change Of Water.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Explain The Process Of Phase Change Of Water The phase change from solid to gas is called. steps of the water cycle: for water, there is no liquid phase at pressures below 0.00600 atm. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. water has the unusual property that ice is less dense than. Explain The Process Of Phase Change Of Water.
From spectrumnews1.com
The amount of water on Earth is a constant Explain The Process Of Phase Change Of Water Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The phase change from solid to gas is called. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. One of these special properties is the fact that solid. steps of the water cycle:. Explain The Process Of Phase Change Of Water.
From schematiclibmammees88.z22.web.core.windows.net
Solid Liquid Phase Diagram Explain The Process Of Phase Change Of Water The phase change from solid to gas is called. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Water is a unique substance in many ways. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from.. Explain The Process Of Phase Change Of Water.
From www.youtube.com
phase changes & water cycle YouTube Explain The Process Of Phase Change Of Water for water, there is no liquid phase at pressures below 0.00600 atm. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. steps of the water cycle: water has the unusual property that ice is less dense than liquid water at the melting point, so at. Explain The Process Of Phase Change Of Water.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download ID6877497 Explain The Process Of Phase Change Of Water One of these special properties is the fact that solid. these changes of state are essential aspects of our earth’s water cycle as well as many other natural phenomena and. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Water is a unique substance in many ways. . Explain The Process Of Phase Change Of Water.