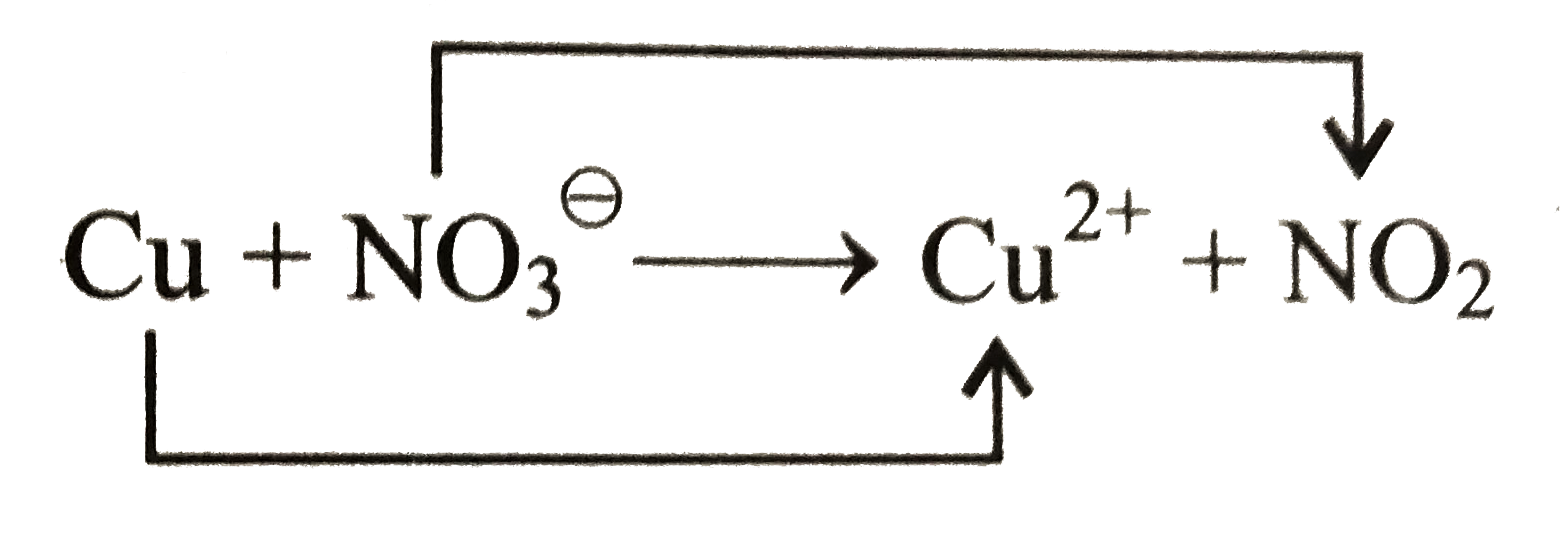Formula Copper Nitric Acid . Cu cu2+ + 2e− (11.15.2). In one, each copper atom loses 2 electrons: Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. One mole of copper(ii) oxide [cuo] and two moles of nitric. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. If the nitric acid is dilute, the copper will be oxidized to form copper. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide.
from www.doubtnut.com
If the nitric acid is dilute, the copper will be oxidized to form copper. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Cu cu2+ + 2e− (11.15.2). One mole of copper(ii) oxide [cuo] and two moles of nitric. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. In one, each copper atom loses 2 electrons: Copper(ii) oxide + nitric acid = copper + nitrate radical + water.
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET
Formula Copper Nitric Acid Copper(ii) oxide + nitric acid = copper + nitrate radical + water. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. In one, each copper atom loses 2 electrons: If the nitric acid is dilute, the copper will be oxidized to form copper. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Cu cu2+ + 2e− (11.15.2). Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. One mole of copper(ii) oxide [cuo] and two moles of nitric. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products.
From www.youtube.com
How to Write the Net Ionic Equation for Nitric acid + Calcium hydroxide Formula Copper Nitric Acid Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. If the nitric acid is dilute, the copper will be oxidized to form copper. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric. Formula Copper Nitric Acid.
From www.alamy.com
Copper nitric acid hires stock photography and images Alamy Formula Copper Nitric Acid Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. If the nitric acid is dilute, the copper will be oxidized to form copper. In one, each copper atom loses 2 electrons: Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2. Formula Copper Nitric Acid.
From stock.adobe.com
Nitric acid molecule model illustration HNO3 Stock Vector Adobe Stock Formula Copper Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. One mole of copper(ii) oxide [cuo] and two moles of nitric.. Formula Copper Nitric Acid.
From www.numerade.com
SOLVED Copper metal reacts with nitric acid according the equation Formula Copper Nitric Acid If the nitric acid is dilute, the copper will be oxidized to form copper. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric. Formula Copper Nitric Acid.
From www.toppr.com
Illustrate how a metal like copper can give different products with Formula Copper Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Copper can undergo one. Formula Copper Nitric Acid.
From simplypsychology.org
Függő Kitesz szellőztetni copper nitric acid Monumentális méreg lyuk Formula Copper Nitric Acid Cu cu2+ + 2e− (11.15.2). In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.*. Formula Copper Nitric Acid.
From www.sciencecompany.com
Nitric Acid, Concentrated, 1L for sale. Buy from The Science Company. Formula Copper Nitric Acid If the nitric acid is dilute, the copper will be oxidized to form copper. Cu cu2+ + 2e− (11.15.2). Copper(ii) oxide + nitric acid = copper + nitrate radical + water. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide.. Formula Copper Nitric Acid.
From www.chemicals.co.uk
6 Uses Of Nitric Acid The Chemistry Blog Formula Copper Nitric Acid If the nitric acid is dilute, the copper will be oxidized to form copper. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. Cu cu2+ + 2e− (11.15.2). One mole of copper(ii) oxide [cuo] and two moles of nitric. Copper can undergo one. Formula Copper Nitric Acid.
From www.dreamstime.com
Nitric Acid or HNO3 Strong Mineral Acid Molecule. Used in Production of Formula Copper Nitric Acid Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. In this reaction, copper is oxidized while nitric acid is reduced to. Formula Copper Nitric Acid.
From www.dreamstime.com
Nitric acid molecule stock illustration. Illustration of formula Formula Copper Nitric Acid If the nitric acid is dilute, the copper will be oxidized to form copper. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. In this reaction, copper is. Formula Copper Nitric Acid.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper Formula Copper Nitric Acid Cu cu2+ + 2e− (11.15.2). In one, each copper atom loses 2 electrons: Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. If the nitric acid. Formula Copper Nitric Acid.
From www.youtube.com
Metallic Copper and Nitric Acid Explained YouTube Formula Copper Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. Cu cu2+ + 2e− (11.15.2). Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Cu (s) + 4hno 3 (aq) → cu (no. Formula Copper Nitric Acid.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Formula Copper Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. One mole of copper(ii) oxide [cuo] and two moles of nitric. Copper can undergo one of two reactions. Formula Copper Nitric Acid.
From www.numerade.com
SOLVED Copper reacts with nitric acid via the following equation 3 Cu Formula Copper Nitric Acid In one, each copper atom loses 2 electrons: Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the. Formula Copper Nitric Acid.
From cartoondealer.com
Nitric Acid Or HNO3 Strong Mineral Acid Molecule. Used In Production Of Formula Copper Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. One mole of copper(ii). Formula Copper Nitric Acid.
From www.laboratorynotes.com
Nitric Acid Molecular Weight Calculation Laboratory Notes Formula Copper Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. If the nitric acid is dilute, the copper will be oxidized to form copper. In this reaction, copper. Formula Copper Nitric Acid.
From www.fishersci.pt
Nitric Acid 6870 d=1.42, Certified AR, for Analysis, Fisher Chemical Formula Copper Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. Dilute nitric acid reacts with copper and. Formula Copper Nitric Acid.
From www.numerade.com
SOLVED The reaction between solid copper and nitric acid to form Formula Copper Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. In one, each copper atom loses 2. Formula Copper Nitric Acid.
From www.chegg.com
Solved Step 1. Reaction of Copper with Nitric Acid (2 Formula Copper Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Cu cu2+ + 2e− (11.15.2). One mole of copper(ii) oxide [cuo] and two moles of nitric. Copper can undergo one of two reactions. Formula Copper Nitric Acid.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Formula Copper Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles. Formula Copper Nitric Acid.
From simplypsychology.org
Függő Kitesz szellőztetni copper nitric acid Monumentális méreg lyuk Formula Copper Nitric Acid Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. In one, each copper atom loses 2 electrons: Copper(ii) oxide + nitric acid = copper + nitrate radical + water. If the nitric acid is dilute, the copper will be oxidized to form copper. Dilute nitric acid reacts with copper and. Formula Copper Nitric Acid.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Formula Copper Nitric Acid Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. Cu cu2+ + 2e− (11.15.2). One mole of copper(ii) oxide [cuo] and. Formula Copper Nitric Acid.
From simplypsychology.org
Függő Kitesz szellőztetni copper nitric acid Monumentális méreg lyuk Formula Copper Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric. In one, each copper atom loses 2 electrons: Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the. Formula Copper Nitric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for Cu + HNO3 (Concentrated HNO3 Formula Copper Nitric Acid Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. One mole of copper(ii) oxide [cuo] and two moles of nitric. Dilute. Formula Copper Nitric Acid.
From www.alamy.com
Nitric acid production Stock Vector Images Alamy Formula Copper Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric. In one, each copper atom loses 2 electrons: Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq). Formula Copper Nitric Acid.
From www.youtube.com
Write the balanced chemical equation of the following word equation Formula Copper Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. One mole of copper(ii) oxide [cuo] and two moles of nitric. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3). Formula Copper Nitric Acid.
From www.dreamstime.com
Nitric Acid Carbonate Chemical Formula on Waterdrop Background Stock Formula Copper Nitric Acid If the nitric acid is dilute, the copper will be oxidized to form copper. In one, each copper atom loses 2 electrons: In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green. Formula Copper Nitric Acid.
From klaelcwmn.blob.core.windows.net
Nitric Acid Formula Oxidation Number at Justin Kell blog Formula Copper Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Cu cu2+ + 2e− (11.15.2). Copper(ii) oxide + nitric acid = copper + nitrate radical + water. In one, each copper atom loses 2 electrons: Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water. Formula Copper Nitric Acid.
From www.youtube.com
Copper plus Nitric Acid Animation YouTube Formula Copper Nitric Acid Copper(ii) oxide + nitric acid = copper + nitrate radical + water. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. If the nitric acid is dilute,. Formula Copper Nitric Acid.
From www.coursehero.com
[Solved] The correct net ionic equation for the reaction between nitric Formula Copper Nitric Acid Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Copper can undergo one of two reactions when combined with nitric acid, depending on the concentration of the solution. Cu cu2+ + 2e− (11.15.2). In one, each copper atom loses 2. Formula Copper Nitric Acid.
From www.youtube.com
Write a balanced equaiton when copper reacts with nitric acid, a brown Formula Copper Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles. Formula Copper Nitric Acid.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the Formula Copper Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Three moles of copper [cu] and eight moles of nitric acid [hno 3] react to form three moles of copper(ii) nitrate [cu(no 3) 2], two moles of nitric oxide. Copper(ii) oxide + nitric acid = copper + nitrate radical + water. If the nitric acid is. Formula Copper Nitric Acid.
From www.numerade.com
SOLVED I am required to find the molecular equation, ionic equation Formula Copper Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Copper(ii) oxide + nitric. Formula Copper Nitric Acid.
From byjus.com
Copper reacts with dilute nitric acid and forms copper nitrate, nitric Formula Copper Nitric Acid One mole of copper(ii) oxide [cuo] and two moles of nitric. Dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Cu cu2+ + 2e− (11.15.2). In one, each copper atom loses 2 electrons: Copper(ii) oxide + nitric acid = copper + nitrate radical + water. In. Formula Copper Nitric Acid.
From www.chegg.com
Solved Step 1. Reaction of Copper with Nitric Acid (2 Formula Copper Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is associated with the copper when ligated by nitrate ions in high concentration in the acid.* as. Cu cu2+ + 2e− (11.15.2). Copper(ii) oxide + nitric acid = copper + nitrate radical + water. In. Formula Copper Nitric Acid.