Acetic Acid Reacts With Sodium Bicarbonate . These 2 components react in. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. The balanced chemical equation is: One of those principles is stoichiometry: Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Nahco3 + ch3cooh → co2 + h2o +. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide.
from www.numerade.com
One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. Nahco3 + ch3cooh → co2 + h2o +. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. One of those principles is stoichiometry: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The balanced chemical equation is: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,.
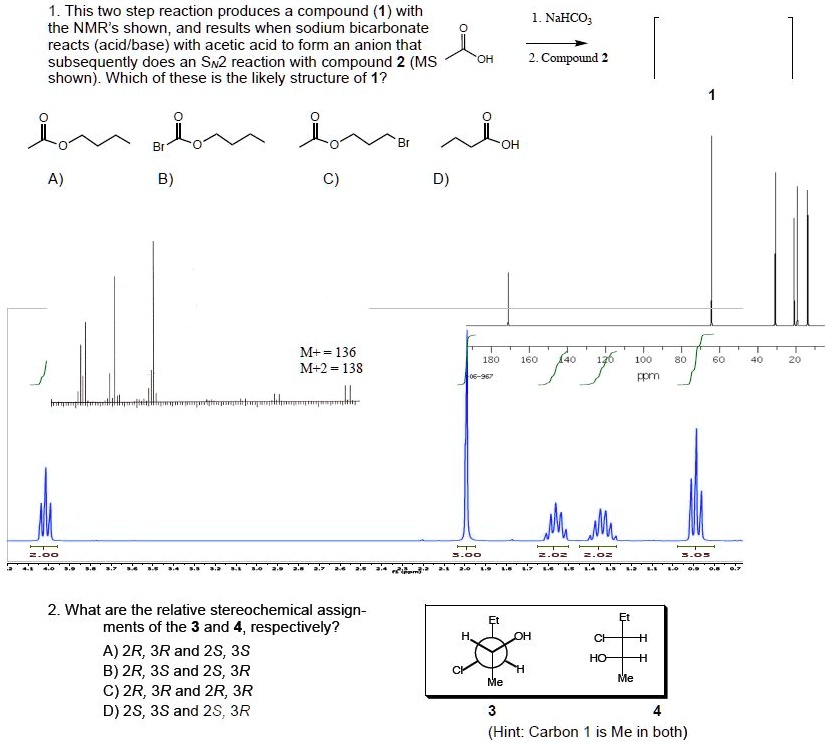
SOLVED This two step reaction produces compound with the NMRs shown
Acetic Acid Reacts With Sodium Bicarbonate Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Nahco3 + ch3cooh → co2 + h2o +. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: The balanced chemical equation is: Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. One of those principles is stoichiometry: These 2 components react in.
From www.coursehero.com
[Solved] . 6. Acetic acid reacts with bicarbonate ion to produce Acetic Acid Reacts With Sodium Bicarbonate The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. One of those principles is stoichiometry: The balanced chemical equation is: These 2 components react in. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3. Acetic Acid Reacts With Sodium Bicarbonate.
From www.numerade.com
SOLVED Acetic acid reacts with sodium carbonate to produce sodium Acetic Acid Reacts With Sodium Bicarbonate One of those principles is stoichiometry: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: The balanced chemical equation is: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2. Acetic Acid Reacts With Sodium Bicarbonate.
From www.numerade.com
SOLVED Write a balanced molecular equation that describes the reaction Acetic Acid Reacts With Sodium Bicarbonate Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Nahco3 + ch3cooh → co2 + h2o. Acetic Acid Reacts With Sodium Bicarbonate.
From askfilo.com
Acetic acid reacts with solid sodium hydrogen carbonate, [2011] ..[1M].. Acetic Acid Reacts With Sodium Bicarbonate These 2 components react in. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The balanced chemical equation is: One mole of sodium bicarbonate. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Chemical Reaction Of Baking Soda And Vinegar (Sodium Bicarbonate And Acetic Acid Reacts With Sodium Bicarbonate The balanced chemical equation is: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react in. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous. Acetic Acid Reacts With Sodium Bicarbonate.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Acetic Acid Reacts With Sodium Bicarbonate One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. These 2 components react in. One of those principles is stoichiometry: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Sodium. Acetic Acid Reacts With Sodium Bicarbonate.
From slideplayer.com
Chemical Reactions. ppt download Acetic Acid Reacts With Sodium Bicarbonate Nahco3 + ch3cooh → co2 + h2o +. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. These 2 components react in. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
sodium bicarbonateacetic acid reaction YouTube Acetic Acid Reacts With Sodium Bicarbonate One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. The balanced chemical equation is: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Nahco 3 + hc 2 h 3. Acetic Acid Reacts With Sodium Bicarbonate.
From www.chegg.com
Solved Reaction 1 Acetic acid and sodium bicarbonate Acetic Acid Reacts With Sodium Bicarbonate One of those principles is stoichiometry: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. The balanced chemical equation is: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2. Acetic Acid Reacts With Sodium Bicarbonate.
From studyx.ai
Solution By Steps Step 1 Setting Up the StudyX Acetic Acid Reacts With Sodium Bicarbonate One of those principles is stoichiometry: Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Nahco3 + ch3cooh → co2 + h2o +. The chemical equation for the reaction between sodium. Acetic Acid Reacts With Sodium Bicarbonate.
From ar.inspiredpencil.com
Sodium Bicarbonate And Acetic Acid Acetic Acid Reacts With Sodium Bicarbonate The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Acetic Acid + Sodium Carbonate = ??? YouTube Acetic Acid Reacts With Sodium Bicarbonate Nahco3 + ch3cooh → co2 + h2o +. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one. Acetic Acid Reacts With Sodium Bicarbonate.
From braidenkruwkirk.blogspot.com
Acetic Acid React With Sodium Carbonate BraidenkruwKirk Acetic Acid Reacts With Sodium Bicarbonate The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Nahco3 + ch3cooh → co2 + h2o +. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. One mole. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Acetic acid and sodium bicarbonate AcidBase reaction YouTube Acetic Acid Reacts With Sodium Bicarbonate Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. The balanced chemical equation is: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. One mole of sodium bicarbonate. Acetic Acid Reacts With Sodium Bicarbonate.
From ar.inspiredpencil.com
Sodium Bicarbonate And Acetic Acid Acetic Acid Reacts With Sodium Bicarbonate Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react in. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Acetic Acid and Sodium bicarbonate Lab YouTube Acetic Acid Reacts With Sodium Bicarbonate The balanced chemical equation is: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Nahco3 + ch3cooh → co2 + h2o +. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. One mole of sodium bicarbonate (baking soda) reacts with. Acetic Acid Reacts With Sodium Bicarbonate.
From slideplayer.com
Reactions and Equations ppt download Acetic Acid Reacts With Sodium Bicarbonate The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from. Acetic Acid Reacts With Sodium Bicarbonate.
From ar.inspiredpencil.com
Sodium Bicarbonate And Acetic Acid Acetic Acid Reacts With Sodium Bicarbonate One of those principles is stoichiometry: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The balanced chemical equation is: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. The chemical equation for the reaction between sodium bicarbonate and acetic. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Acetic acid + sodium bicarbonate YouTube Acetic Acid Reacts With Sodium Bicarbonate Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. These 2 components react in. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: The balanced. Acetic Acid Reacts With Sodium Bicarbonate.
From www.numerade.com
SOLVED Acetic acid and sodium bicarbonate are reacted and the gas Acetic Acid Reacts With Sodium Bicarbonate One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Baking soda is a. Acetic Acid Reacts With Sodium Bicarbonate.
From ar.inspiredpencil.com
Sodium Bicarbonate And Acetic Acid Acetic Acid Reacts With Sodium Bicarbonate The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. One of those principles is stoichiometry: The balanced chemical equation is: Nahco3. Acetic Acid Reacts With Sodium Bicarbonate.
From www.scribd.com
Reactions of Acetic Acid and Sodium Bicarbonate Sodium Bicarbonate Acetic Acid Reacts With Sodium Bicarbonate Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. One of those principles is stoichiometry: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one. Acetic Acid Reacts With Sodium Bicarbonate.
From www.numerade.com
SOLVED This two step reaction produces compound with the NMRs shown Acetic Acid Reacts With Sodium Bicarbonate Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react in. Nahco3 + ch3cooh → co2 + h2o +. The balanced chemical equation is: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Nahco 3 + hc 2 h 3 o 2 → nac 2. Acetic Acid Reacts With Sodium Bicarbonate.
From ar.inspiredpencil.com
Sodium Bicarbonate And Acetic Acid Acetic Acid Reacts With Sodium Bicarbonate Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. The chemical equation for. Acetic Acid Reacts With Sodium Bicarbonate.
From www.alamy.com
Chemical Reaction of Baking Soda (sodium bicarbonate) and Vinegar Acetic Acid Reacts With Sodium Bicarbonate Nahco3 + ch3cooh → co2 + h2o +. One of those principles is stoichiometry: The balanced chemical equation is: Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. Nahco 3 + hc 2 h. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Acetic Acid and Sodium Bicarbonate (vinegar and baking soda) Chemical Acetic Acid Reacts With Sodium Bicarbonate These 2 components react in. The balanced chemical equation is: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Nahco3 + ch3cooh → co2 + h2o +. One of those principles is stoichiometry: The chemical equation for the reaction between sodium bicarbonate and acetic acid is. Acetic Acid Reacts With Sodium Bicarbonate.
From thomasyouthhouse.blogspot.com
Sodium Bicarbonate and Acetic Acid Balanced Chemical Equation Acetic Acid Reacts With Sodium Bicarbonate One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. One of those principles is stoichiometry: The chemical equation for the reaction. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Acetic acid and sodium bicarbonate YouTube Acetic Acid Reacts With Sodium Bicarbonate Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. These 2 components react in. The balanced. Acetic Acid Reacts With Sodium Bicarbonate.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium Acetic Acid Reacts With Sodium Bicarbonate The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: One of those principles is stoichiometry: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react in. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2. Acetic Acid Reacts With Sodium Bicarbonate.
From www.numerade.com
SOLVED Aqueous solutions of sodium bicarbonate and sulfuric acid react Acetic Acid Reacts With Sodium Bicarbonate One of those principles is stoichiometry: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. Nahco 3 + hc 2 h. Acetic Acid Reacts With Sodium Bicarbonate.
From www.numerade.com
SOLVED Write the balanced equation for the reaction of acetic acid Acetic Acid Reacts With Sodium Bicarbonate The balanced chemical equation is: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o. Acetic Acid Reacts With Sodium Bicarbonate.
From fineartamerica.com
Reaction Of Sodium Carbonate In Acid Photograph by Martyn F. Chillmaid Acetic Acid Reacts With Sodium Bicarbonate These 2 components react in. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: One of those principles is stoichiometry: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Nahco3 + ch3cooh → co2 + h2o +. The balanced chemical. Acetic Acid Reacts With Sodium Bicarbonate.
From www.bartleby.com
Answered 20LL 2.Write a net ionic equation for… bartleby Acetic Acid Reacts With Sodium Bicarbonate Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. Nahco3 + ch3cooh → co2 + h2o +. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic. Acetic Acid Reacts With Sodium Bicarbonate.
From www.youtube.com
Reaction of sodium bicarbonate( baking soda or commonly known as soda Acetic Acid Reacts With Sodium Bicarbonate Nahco3 + ch3cooh → co2 + h2o +. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. Sodium bicarbonate, nahco3, will react with acetic acid, ch3cooh, to produce aqueous sodium acetate, ch3coona,. The balanced chemical equation. Acetic Acid Reacts With Sodium Bicarbonate.
From www.coursehero.com
[Solved] Beaker B contains 5 ml of acetic acid, 0.5 g of sodium Acetic Acid Reacts With Sodium Bicarbonate Nahco3 + ch3cooh → co2 + h2o +. The balanced chemical equation is: One of those principles is stoichiometry: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react in. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o +. Acetic Acid Reacts With Sodium Bicarbonate.