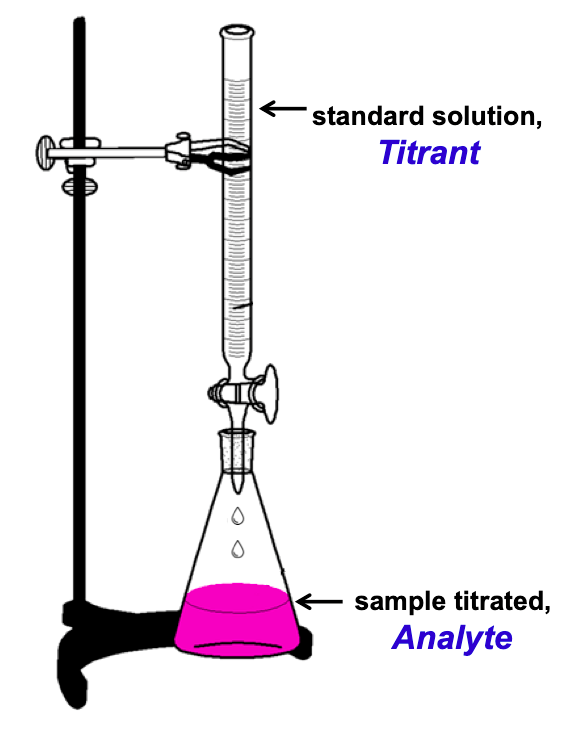
What Is An Analyte Solution . A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. It can be a simple. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The titrant is the known solution which has a precise and. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The analyte (titrand) is the solution with an unknown molarity. The analyte is the unknown solution for which you would like to know either the concentration or the equilibrium constant. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality.
from wisc.pb.unizin.org
The analyte is the unknown solution for which you would like to know either the concentration or the equilibrium constant. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. The titrant is the known solution which has a precise and. The analyte (titrand) is the solution with an unknown molarity. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It can be a simple. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104
What Is An Analyte Solution A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. The analyte is the unknown solution for which you would like to know either the concentration or the equilibrium constant. The analyte (titrand) is the solution with an unknown molarity. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The titrant is the known solution which has a precise and. It can be a simple.
From www.chegg.com
Solved Method of Standard Addition Determining Analyte What Is An Analyte Solution The titrant is the known solution which has a precise and. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. The analyte is the unknown solution for which you would like to. What Is An Analyte Solution.
From www.youtube.com
MEI FPT Differential Equations 2 Analytical solutions Worked What Is An Analyte Solution A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added. What Is An Analyte Solution.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps What Is An Analyte Solution It can be a simple. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A constant electrochemical potential suitable. What Is An Analyte Solution.
From www.researchgate.net
Recovered Hg(0) (2) from the analyte solution (1). Download What Is An Analyte Solution Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. A titration is a volumetric technique in which a. What Is An Analyte Solution.
From www.gauthmath.com
Solved Related to titration, what is an analyte? The point during a What Is An Analyte Solution The analyte (titrand) is the solution with an unknown molarity. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and. What Is An Analyte Solution.
From www.chegg.com
Solved You have a solution of an analyte with a molar What Is An Analyte Solution A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. Titration is a fast, accurate and established analytical technique. What Is An Analyte Solution.
From hxerxlalm.blob.core.windows.net
What Is The Initial Ph Of The Analyte Solution at Lloyd Bone blog What Is An Analyte Solution A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. It can be a simple. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. What Is An Analyte Solution.
From www.slideserve.com
PPT ERT207 Analytical Chemistry Gravimetric Analysis and What Is An Analyte Solution Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. The analyte (titrand) is the solution with an unknown. What Is An Analyte Solution.
From www.slideserve.com
PPT ERT207 Analytical Chemistry Gravimetric Analysis and What Is An Analyte Solution Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A. What Is An Analyte Solution.
From studylib.net
Introduction What Is An Analyte Solution Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It. What Is An Analyte Solution.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 What Is An Analyte Solution Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. The titrant is the known solution which has a precise and. The analyte (titrand) is the solution with an unknown molarity. A titration is a volumetric technique in. What Is An Analyte Solution.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? What Is An Analyte Solution Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is the known solution which has a precise and. A constant electrochemical. What Is An Analyte Solution.
From www.gauthmath.com
Related to titration, what is an analyte? A. The point during a What Is An Analyte Solution The analyte (titrand) is the solution with an unknown molarity. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and. What Is An Analyte Solution.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145156 What Is An Analyte Solution In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. The analyte (titrand) is. What Is An Analyte Solution.
From slideplayer.com
Conservation of Atoms Main Concept ppt download What Is An Analyte Solution The titrant is the known solution which has a precise and. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It can be. What Is An Analyte Solution.
From www.chegg.com
Solved What is the analyte in an acidbase titration What Is An Analyte Solution The analyte (titrand) is the solution with an unknown molarity. The titrant is the known solution which has a precise and. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. The analyte is the unknown solution for which you would like to know either the concentration or the equilibrium constant.. What Is An Analyte Solution.
From www.aquaportail.com
Analyte définition et explications What Is An Analyte Solution It can be a simple. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. What Is An Analyte Solution.
From www.slideserve.com
PPT Introduction to Biosensors PowerPoint Presentation ID219807 What Is An Analyte Solution It can be a simple. The titrant is the known solution which has a precise and. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Titration involves the gradual addition of a. What Is An Analyte Solution.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation What Is An Analyte Solution Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It. What Is An Analyte Solution.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom What Is An Analyte Solution Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The titrant is the known solution which has a. What Is An Analyte Solution.
From www.chegg.com
Solved Two different analyte solutions of the same acid, What Is An Analyte Solution Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant (the. What Is An Analyte Solution.
From www.microlit.com
An Advanced Guide to Titration Microlit What Is An Analyte Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The analyte (titrand) is the solution with an unknown molarity. The analyte is the unknown solution for which you would like to know either the concentration or the equilibrium constant. Titration is a. What Is An Analyte Solution.
From www.bigstockphoto.com
Solution Infographic Image & Photo (Free Trial) Bigstock What Is An Analyte Solution The analyte (titrand) is the solution with an unknown molarity. The titrant is the known solution which has a precise and. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration. What Is An Analyte Solution.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID What Is An Analyte Solution The analyte is the unknown solution for which you would like to know either the concentration or the equilibrium constant. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a fast, accurate and established analytical technique that is widely applied. What Is An Analyte Solution.
From www.chegg.com
Solved A 50.00 mL sample containing an analyte gives a What Is An Analyte Solution A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. It can be a simple. Titration is a fast,. What Is An Analyte Solution.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school What Is An Analyte Solution A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. The analyte (titrand) is the solution with an unknown molarity. The titrant is the known solution which has a precise and. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 +. What Is An Analyte Solution.
From slideplayer.com
Conservation of Atoms Main Concept ppt download What Is An Analyte Solution Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. The analyte (titrand) is the solution with an unknown molarity. The titrant is the known solution which has a precise and. In analytical chemistry, an analyte is defined. What Is An Analyte Solution.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog What Is An Analyte Solution The analyte (titrand) is the solution with an unknown molarity. Rather than performing the titration by adding the ce 4 + from a burette, an excess of ce 3 + is added to an accurately measured volume of the unknown analyte solution. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and. What Is An Analyte Solution.
From www.slideshare.net
Acid base titration What Is An Analyte Solution It can be a simple. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. Titration is a fast, accurate and established analytical technique that. What Is An Analyte Solution.
From www.youtube.com
Analytical solution of 2D Laplace equation YouTube What Is An Analyte Solution The analyte (titrand) is the solution with an unknown molarity. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The analyte is the unknown solution for which you would like to know either the concentration or the. What Is An Analyte Solution.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool What Is An Analyte Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It can be a simple. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. A titration is a volumetric technique in which a solution. What Is An Analyte Solution.
From www.slideserve.com
PPT Differential Equations PowerPoint Presentation, free download What Is An Analyte Solution The titrant is the known solution which has a precise and. A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. It can be a simple. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. What Is An Analyte Solution.
From www.pinterest.com
How to Calculate Normality of a Solution Chemistry lessons, Chemistry What Is An Analyte Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. Rather than performing the titration by adding the ce 4 + from a burette, an. What Is An Analyte Solution.
From www.chegg.com
Solved Which statement is true given the experimental What Is An Analyte Solution It can be a simple. A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. Titration is a fast, accurate and established analytical technique that is widely applied in research, product development, and quality. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. What Is An Analyte Solution.
From www.slideserve.com
PPT Titrimetry PowerPoint Presentation, free download ID2261229 What Is An Analyte Solution A constant electrochemical potential suitable to convert ce 3 + to ce 4 + is applied to the solution. The titrant is the known solution which has a precise and. In analytical chemistry, an analyte is defined as the substance of interest, the chemical species subject to analysis. Titration is a fast, accurate and established analytical technique that is widely. What Is An Analyte Solution.