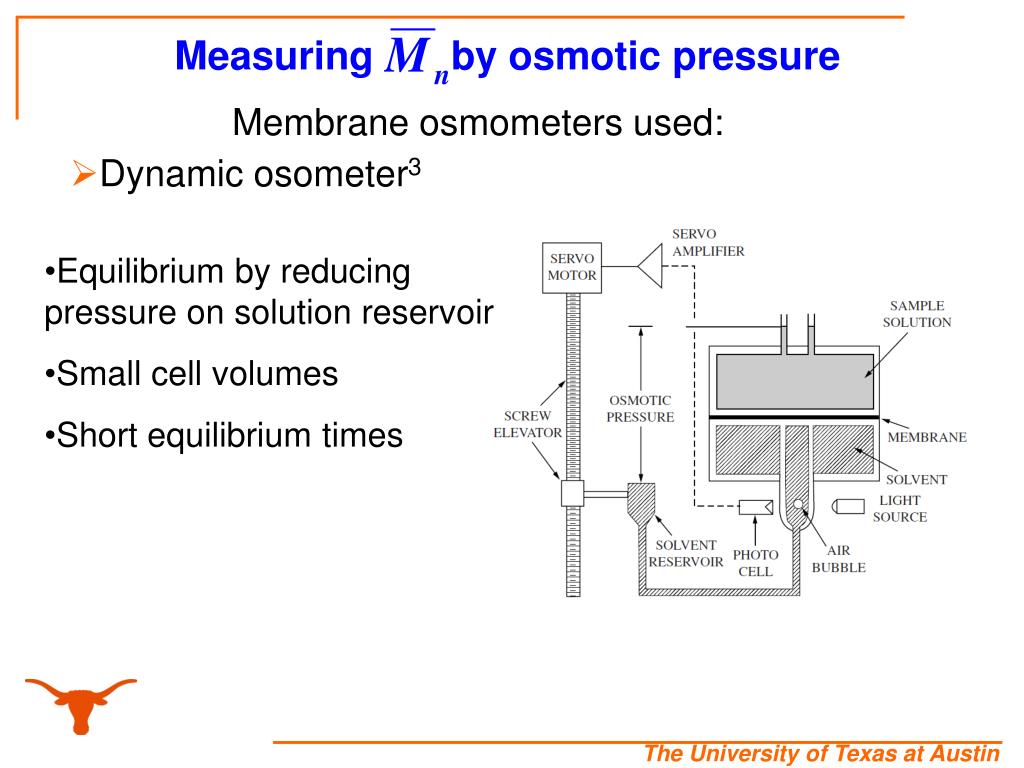
Osmometry Slideshare . A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Types of osmometers and their operating principle. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry measures the osmotic strength of a solution using an. [1] membrane osmometry uses a. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolality is the concentration of osmoles in a. Most modern osmometers share a common principle: Anurag yadav presented on osmometry and osmolality. There are two types of osmometer: Osmometry is applied to determine number average of molecular weight (m n).
from www.slideserve.com
A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Anurag yadav presented on osmometry and osmolality. An osmole is 1 mole of any fully dissociated substance dissolved in water. [1] membrane osmometry uses a. There are two types of osmometer: Osmometry measures the osmotic strength of a solution using an. Most modern osmometers share a common principle: Osmometry is applied to determine number average of molecular weight (m n). Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmolality is the concentration of osmoles in a.
PPT Molecular Weight and polymer properties Methods Used to determine
Osmometry Slideshare Osmometry measures the osmotic strength of a solution using an. Osmometry is applied to determine number average of molecular weight (m n). Most modern osmometers share a common principle: The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. [1] membrane osmometry uses a. Types of osmometers and their operating principle. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Anurag yadav presented on osmometry and osmolality. Osmometry measures the osmotic strength of a solution using an. An osmole is 1 mole of any fully dissociated substance dissolved in water. There are two types of osmometer: Osmolality is the concentration of osmoles in a.
From www.scribd.com
Vapor Pressure Osmometry PDF Osmometry Slideshare There are two types of osmometer: [1] membrane osmometry uses a. Types of osmometers and their operating principle. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmometry is applied to determine number average of molecular weight (m n). Osmometry measures the osmotic strength of. Osmometry Slideshare.
From www.purechemistry.org
Osmometry method to determine molecular weight of polymer Purechemistry Osmometry Slideshare Most modern osmometers share a common principle: Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. A relative technique used to determine the number average molecular weight (mn). Osmometry Slideshare.
From www.biophlox.com
A Complete Lab Guide to Osmometer Osmometry Slideshare An osmole is 1 mole of any fully dissociated substance dissolved in water. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry measures the osmotic strength of a solution using an. Types of osmometers and their operating principle. Osmometry is a technique that measures the concentration of solute particles contributing to. Osmometry Slideshare.
From www.chegg.com
Solved 5. Osmometry (see figure below) is an important Osmometry Slideshare The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Anurag yadav presented on osmometry and osmolality. [1] membrane osmometry uses a. An osmole is 1 mole of any fully dissociated substance dissolved in water. Most modern osmometers share a common principle: Types of osmometers and their operating principle. Osmometry is a technique. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare [1] membrane osmometry uses a. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Types of osmometers and their operating principle. An osmole is 1. Osmometry Slideshare.
From www.studypool.com
SOLUTION Demonstration of osmosis by potato osmometer presentation Osmometry Slideshare Osmometry is applied to determine number average of molecular weight (m n). Anurag yadav presented on osmometry and osmolality. Most modern osmometers share a common principle: Osmometry measures the osmotic strength of a solution using an. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. An osmole is 1. Osmometry Slideshare.
From www.slideserve.com
PPT Labs 6 & 7 PowerPoint Presentation, free download ID2180146 Osmometry Slideshare The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolality is the concentration of osmoles in a. Anurag yadav presented on osmometry and osmolality. A relative technique used to determine the number average molecular weight (mn) of a polymer. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Osmometry measures the osmotic strength of a solution using an. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Types of osmometers and their operating. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare Most modern osmometers share a common principle: Osmolality is the concentration of osmoles in a. Types of osmometers and their operating principle. [1] membrane osmometry uses a. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmometry measures the osmotic strength of a solution using. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare Most modern osmometers share a common principle: Anurag yadav presented on osmometry and osmolality. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmometry measures the osmotic strength of a solution using. Osmometry Slideshare.
From www.pinterest.com
An osmometer can use any of the following principles except Osmometry Slideshare Osmometry is applied to determine number average of molecular weight (m n). Most modern osmometers share a common principle: Osmolality is the concentration of osmoles in a. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. An osmole is 1 mole of any fully dissociated substance dissolved in water.. Osmometry Slideshare.
From www.slideserve.com
PPT Labs 6 & 7 PowerPoint Presentation, free download ID2180146 Osmometry Slideshare Osmolality is the concentration of osmoles in a. There are two types of osmometer: [1] membrane osmometry uses a. Osmometry measures the osmotic strength of a solution using an. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Osmometry is a technique that measures the concentration of solute particles. Osmometry Slideshare.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry Slideshare An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmometry measures the osmotic strength of a solution using an. Osmolality is the concentration of osmoles in a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry is applied to determine number average of molecular weight (m n).. Osmometry Slideshare.
From www.slideserve.com
PPT Diffusion and Osmosis PowerPoint Presentation, free download ID Osmometry Slideshare Osmometry measures the osmotic strength of a solution using an. Anurag yadav presented on osmometry and osmolality. There are two types of osmometer: Osmolality is the concentration of osmoles in a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry is applied to determine number average of molecular weight (m n).. Osmometry Slideshare.
From www.scribd.com
Osmometry Electrochemistry PDF Chemical Substances Chemistry Osmometry Slideshare Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmometry measures the osmotic strength of a solution using an. [1] membrane osmometry uses a. Most modern osmometers share a common principle: Anurag. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Osmolality is the concentration of osmoles in a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Types of osmometers and their operating principle. Osmometry measures the osmotic strength of a solution. Osmometry Slideshare.
From www.docsity.com
Osmometry and Light ScatteringPolymer Science and EngineeringLecture Osmometry Slideshare Osmolality is the concentration of osmoles in a. An osmole is 1 mole of any fully dissociated substance dissolved in water. [1] membrane osmometry uses a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Most modern osmometers share a common principle: Osmometry measures the osmotic strength of a solution using an.. Osmometry Slideshare.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Osmometry Slideshare The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmometry is applied to determine number average of molecular weight (m n). Osmometry measures the osmotic strength of a. Osmometry Slideshare.
From slidetodoc.com
Membrane Osmometry Alfredo Clemente CH 392 N Prof Osmometry Slideshare [1] membrane osmometry uses a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry is applied to determine number average of molecular weight (m n). Osmometry measures the osmotic strength of a solution using an. Most modern osmometers share a common principle: A relative technique used to determine the number average. Osmometry Slideshare.
From www.goconqr.com
Movement of Substances into and out of Cells(2Pages) Note Osmometry Slideshare Osmolality is the concentration of osmoles in a. Osmometry is applied to determine number average of molecular weight (m n). There are two types of osmometer: Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. A relative technique used to determine the number average molecular. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare An osmole is 1 mole of any fully dissociated substance dissolved in water. Most modern osmometers share a common principle: Anurag yadav presented on osmometry and osmolality. Osmometry measures the osmotic strength of a solution using an. Types of osmometers and their operating principle. [1] membrane osmometry uses a. Osmolality is the concentration of osmoles in a. Osmometry is a. Osmometry Slideshare.
From www.slideshare.net
Osmometry report.pdf Osmometry Slideshare Osmometry is applied to determine number average of molecular weight (m n). [1] membrane osmometry uses a. Types of osmometers and their operating principle. There are two types of osmometer: A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Anurag yadav presented on osmometry and osmolality. Osmolality is the. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmolality is the concentration of osmoles in a. Types of osmometers and their operating principle. Osmometry measures the osmotic strength of a solution. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry is applied to determine number average of molecular weight (m n). Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. An osmole is 1 mole of any. Osmometry Slideshare.
From www.slideserve.com
PPT Clinical Chemistry Chapter 4 PowerPoint Presentation, free Osmometry Slideshare [1] membrane osmometry uses a. Osmometry is applied to determine number average of molecular weight (m n). Osmometry measures the osmotic strength of a solution using an. Most modern osmometers share a common principle: Osmolality is the concentration of osmoles in a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare Osmometry measures the osmotic strength of a solution using an. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmolality is the concentration of osmoles in a. Most modern osmometers share a common principle: A relative technique used to determine the number average molecular weight. Osmometry Slideshare.
From www.slideshare.net
Ept 121 lecture membrane osmometry Osmometry Slideshare [1] membrane osmometry uses a. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmometry is applied to determine number average of molecular weight (m. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare Types of osmometers and their operating principle. Osmolality is the concentration of osmoles in a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. Osmometry measures the osmotic strength of a solution using an. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare Most modern osmometers share a common principle: A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. There are two types of osmometer: Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmolality is the. Osmometry Slideshare.
From www.youtube.com
Osmometry Walkthrough YouTube Osmometry Slideshare Osmometry measures the osmotic strength of a solution using an. Most modern osmometers share a common principle: There are two types of osmometer: Anurag yadav presented on osmometry and osmolality. [1] membrane osmometry uses a. Osmometry is applied to determine number average of molecular weight (m n). Osmolality is the concentration of osmoles in a. A relative technique used to. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare Types of osmometers and their operating principle. Osmolality is the concentration of osmoles in a. Most modern osmometers share a common principle: Osmometry is applied to determine number average of molecular weight (m n). Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. An osmole. Osmometry Slideshare.
From www.scribd.com
osmometry ppt Osmometry Slideshare An osmole is 1 mole of any fully dissociated substance dissolved in water. [1] membrane osmometry uses a. Types of osmometers and their operating principle. Osmolality is the concentration of osmoles in a. Anurag yadav presented on osmometry and osmolality. Osmometry is applied to determine number average of molecular weight (m n). There are two types of osmometer: Most modern. Osmometry Slideshare.
From www.flickr.com
Membrane Osmometry Diagram of a membrane osmometer. Format… Flickr Osmometry Slideshare Osmometry is a technique that measures the concentration of solute particles contributing to the osmotic pressure of a solution by measuring its freezing point. Osmolality is the concentration of osmoles in a. Anurag yadav presented on osmometry and osmolality. Types of osmometers and their operating principle. Most modern osmometers share a common principle: There are two types of osmometer: Osmometry. Osmometry Slideshare.
From studylib.net
Membrane Osmometry ( , A , χ) M 2 Osmometry Slideshare Anurag yadav presented on osmometry and osmolality. [1] membrane osmometry uses a. The document discusses the molecular weight of polymers and methods to determine it, focusing on membrane osmometry. There are two types of osmometer: A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Most modern osmometers share a. Osmometry Slideshare.
From www.slideshare.net
Osmometry by Dr. Anurag Yadav Osmometry Slideshare [1] membrane osmometry uses a. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolality is the concentration of osmoles in a. Most modern osmometers share a common principle: Osmometry is applied to determine number average of molecular weight (m n). There are two types of osmometer: Osmometry measures the osmotic strength of a solution using. Osmometry Slideshare.