Device Regulations Eu . Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. It repeals directive 93/42/eec (mdd), which.
from mavenprofserv.com
It repeals directive 93/42/eec (mdd), which. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions.
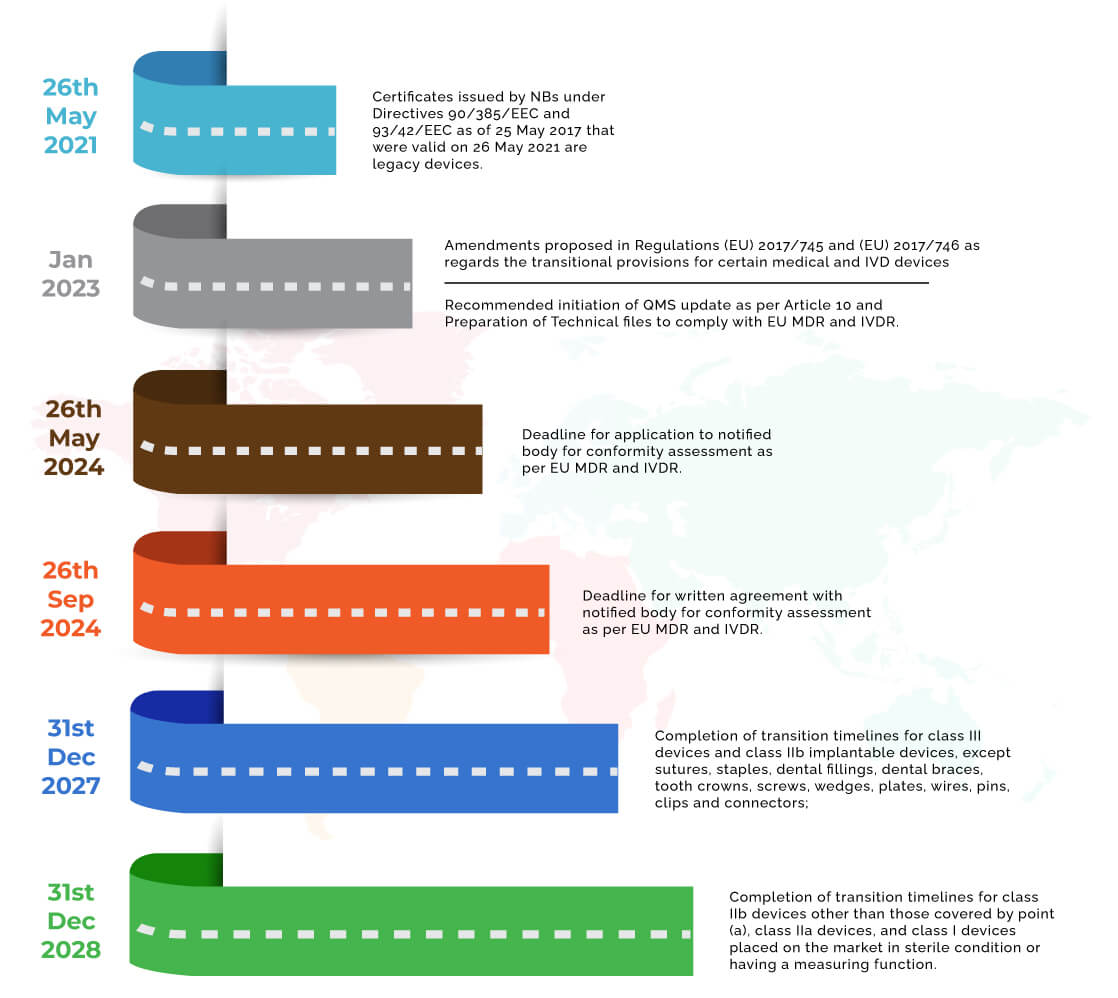
(EU) 2017/745 and (EU) 2017/746 Navigating Timelines
Device Regulations Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. It repeals directive 93/42/eec (mdd), which. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.
From www.vrogue.co
Eu Mdr Medical Device Regulations Timeline vrogue.co Device Regulations Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. It repeals directive 93/42/eec (mdd), which. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Device Regulations Eu.
From somaap.org
Medical device regulation 2024 745, Regulation (EU) 2017/745 Device Regulations Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regul ation (eu). Device Regulations Eu.
From gxp-training.com
Medical Device Regulation MDR 2017/745 Course and Certificate Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. It repeals directive 93/42/eec (mdd), which. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april. Device Regulations Eu.
From www.researchgate.net
(PDF) Medical Device Regulation A Comparison of the United States and Device Regulations Eu Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Device Regulations Eu.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Device Regulations Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/745. Device Regulations Eu.
From www.vrogue.co
Eu Ivd Approval Process For Medical Devices vrogue.co Device Regulations Eu It repeals directive 93/42/eec (mdd), which. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes. Device Regulations Eu.
From www.jamasoftware.com
Takeaways What Changes to the EU MDR Mean for You Jama Software Device Regulations Eu It repeals directive 93/42/eec (mdd), which. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical. Device Regulations Eu.
From www.researchgate.net
(PDF) A Review on European Union New Medical Device Regulations2017 Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. It. Device Regulations Eu.
From gemarmed.com
Getting CE Marking with EU MDR Requirements GEMARMED Device Regulations Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation. Device Regulations Eu.
From www.presentationeze.com
MDR Medical Device Regulation EU 2017 745 Timeline PresentationEZE Device Regulations Eu It repeals directive 93/42/eec (mdd), which. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation. Device Regulations Eu.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Device Regulations Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. It repeals directive 93/42/eec (mdd), which. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746. Device Regulations Eu.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Device Regulations Eu Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745. Device Regulations Eu.
From www.tuv.com
EU Medical Device Regulation MDR 2017/745 SA TÜV Rheinland Device Regulations Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use.. Device Regulations Eu.
From somaap.org
Medical device regulation 2024 745, Regulation (EU) 2017/745 Device Regulations Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745. Device Regulations Eu.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regul. Device Regulations Eu.
From w3inte.intertek.com.mx
New EU Medical Devices Regulation/In Vitro Diagnostics Regulation Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Device Regulations Eu.
From www.stendard.io
6 Major Implementations in the EU Medical Devices Regulation (MDR Device Regulations Eu It repeals directive 93/42/eec (mdd), which. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746. Device Regulations Eu.
From pra-me.com
How the EU Medical Device Regulation will affect the GCC Device Regulations Eu It repeals directive 93/42/eec (mdd), which. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746. Device Regulations Eu.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The. Device Regulations Eu.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Device Regulations Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. It repeals directive 93/42/eec (mdd), which. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr. Device Regulations Eu.
From omcmedical.com
Vigilance Terms & Concepts (EU) 2017/745 on Medical Devices Device Regulations Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. It repeals directive 93/42/eec (mdd), which. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. Device Regulations Eu.
From planetinnovation.com
The EU Medical Device Regulations (EU MDR 2017/745) in a nutshell Device Regulations Eu Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. It repeals directive 93/42/eec (mdd), which. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and. Device Regulations Eu.
From crfweb.com
Medical Device Regulations Device Regulations Eu Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. It repeals directive 93/42/eec (mdd), which. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards. Device Regulations Eu.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Device Regulations Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices,. Device Regulations Eu.
From advanxa.eu
MDR / EUDAMED Advanxa Device Regulations Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It repeals directive 93/42/eec (mdd), which. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017. Device Regulations Eu.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Device Regulations Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 is a. Device Regulations Eu.
From somaap.org
Medical device regulation 2024 745, Regulation (EU) 2017/745 Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regul ation (eu). Device Regulations Eu.
From somaap.org
Medical device regulation 2024 745, Regulation (EU) 2017/745 Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices,. Device Regulations Eu.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Device Regulations Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. It repeals directive 93/42/eec (mdd), which. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. Regul ation (eu) 2017/745 of the european parliament and of the council of 5. Device Regulations Eu.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I Device Regulations Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 is a. Device Regulations Eu.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and Device Regulations Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. It repeals directive 93/42/eec (mdd), which. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Device Regulations Eu.
From mavenprofserv.com
(EU) 2017/745 and (EU) 2017/746 Navigating Timelines Device Regulations Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It repeals directive 93/42/eec (mdd), which. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the. Device Regulations Eu.
From www.hpcosmos.com
Certificates based on MDR Medical Device Regulation (EU) 2017/745 and Device Regulations Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. The. Device Regulations Eu.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Device Regulations Eu Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical devices for human use. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.. Device Regulations Eu.
From www.dreamstime.com
MDR Medical Device Regulation. Regulation of the EU European Union Device Regulations Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Regul ation (eu) 2017/745 of the european parliament and of the council of 5 apr il 2017 on medical devices, amending directive. Regulation (eu) 2017/745 is a regulation of the european union on the clinical investigation and sale of medical. Device Regulations Eu.