Endothermic Reaction Or Product . These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. chemical reactions that absorb (or use) energy overall are called endothermic. an endothermic reaction absorbs heat and cools the environment. A hallmark of this type of reaction is that it feels cold. an endothermic reaction is any chemical reaction that absorbs heat from its environment. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. Investigating endothermic and exothermic reactions aims to estimate energy changes in. A good endothermic reaction example includes dissolving a salt. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In endothermic reactions, more energy is. The absorbed energy provides the activation energy for the reaction to occur.
from www.doubtnut.com
The absorbed energy provides the activation energy for the reaction to occur. In endothermic reactions, more energy is. an endothermic reaction absorbs heat and cools the environment. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. an endothermic reaction is any chemical reaction that absorbs heat from its environment. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. chemical reactions that absorb (or use) energy overall are called endothermic. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A good endothermic reaction example includes dissolving a salt. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
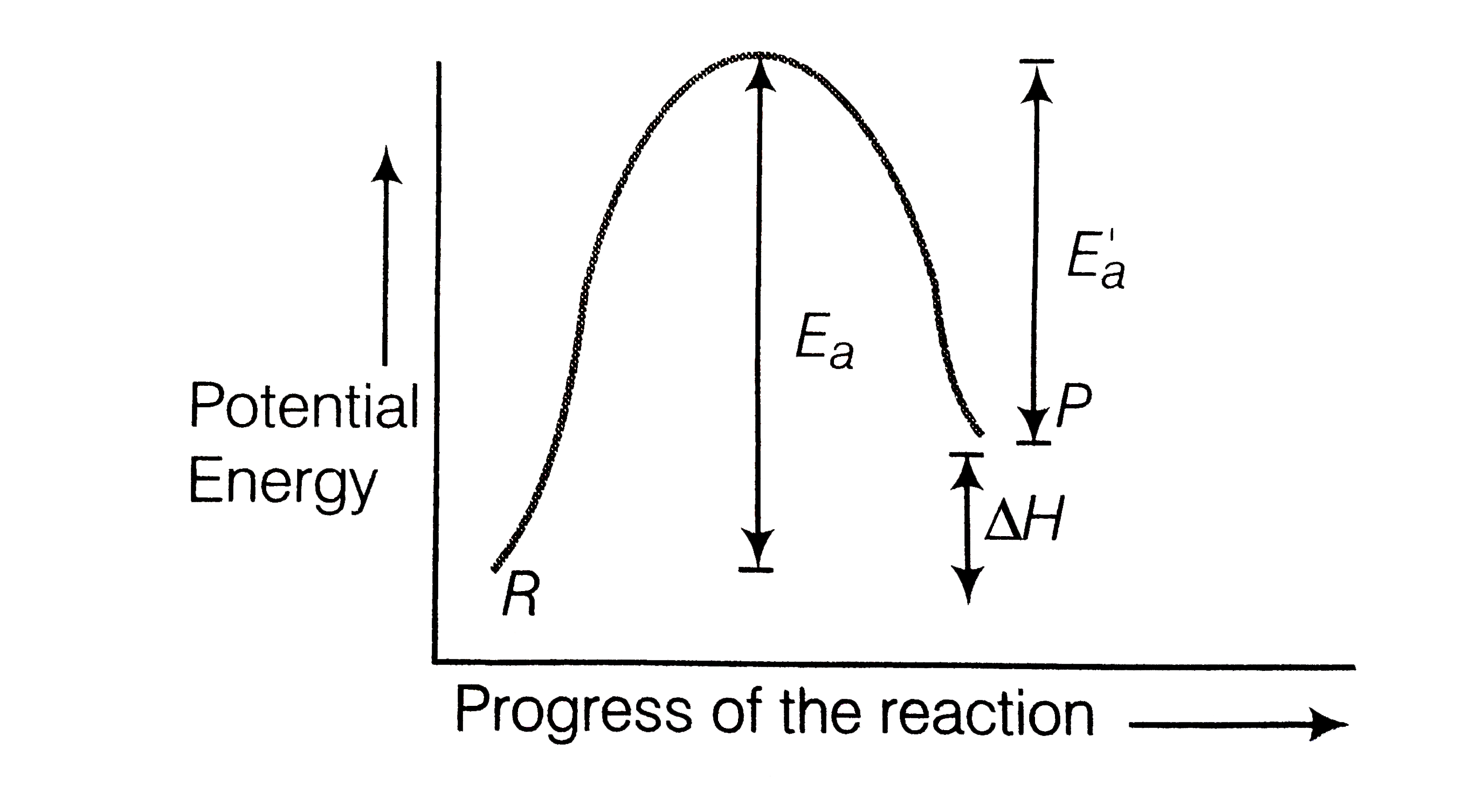
For an endothermic reaction energy of activation is E(a) and enthlpy o
Endothermic Reaction Or Product for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The absorbed energy provides the activation energy for the reaction to occur. In endothermic reactions, more energy is. A hallmark of this type of reaction is that it feels cold. Investigating endothermic and exothermic reactions aims to estimate energy changes in. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction absorbs heat and cools the environment. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. A good endothermic reaction example includes dissolving a salt. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. chemical reactions that absorb (or use) energy overall are called endothermic. an endothermic reaction is any chemical reaction that absorbs heat from its environment.
From www.pinterest.com
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Reaction Or Product In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. Investigating endothermic and exothermic reactions aims to estimate energy changes in. an endothermic reaction absorbs heat and cools the environment. an endothermic reaction is any chemical reaction that absorbs heat from its environment. In endothermic reactions, more energy is. . Endothermic Reaction Or Product.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Or Product The absorbed energy provides the activation energy for the reaction to occur. In endothermic reactions, more energy is. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. an endothermic reaction absorbs heat and cools the environment. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.. Endothermic Reaction Or Product.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Or Product The absorbed energy provides the activation energy for the reaction to occur. chemical reactions that absorb (or use) energy overall are called endothermic. A good endothermic reaction example includes dissolving a salt. an endothermic reaction is any chemical reaction that absorbs heat from its environment. A hallmark of this type of reaction is that it feels cold. . Endothermic Reaction Or Product.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Or Product an endothermic reaction absorbs heat and cools the environment. A hallmark of this type of reaction is that it feels cold. In endothermic reactions, more energy is. an endothermic reaction is any chemical reaction that absorbs heat from its environment. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form. Endothermic Reaction Or Product.
From stock.adobe.com
Vecteur Stock Vector graphs or charts of endothermic and exothermic Endothermic Reaction Or Product A hallmark of this type of reaction is that it feels cold. an endothermic reaction absorbs heat and cools the environment. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form. Endothermic Reaction Or Product.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Or Product In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. In endothermic reactions, more energy is. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. an endothermic reaction is any chemical reaction that absorbs heat from its environment. . Endothermic Reaction Or Product.
From partdiagramaminabakery5v.z14.web.core.windows.net
Exothermic And Endothermic Diagrams Endothermic Reaction Or Product for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A good endothermic reaction example includes dissolving a salt. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. an endothermic reaction is any chemical reaction that absorbs heat. Endothermic Reaction Or Product.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Or Product These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Investigating endothermic and exothermic reactions aims to estimate energy changes in. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. chemical reactions that absorb (or use) energy overall are called endothermic. A hallmark. Endothermic Reaction Or Product.
From www.slideserve.com
PPT Energy Changes PowerPoint Presentation, free download ID2262239 Endothermic Reaction Or Product an endothermic reaction absorbs heat and cools the environment. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. A hallmark of this type of reaction is that it feels cold. A good endothermic reaction example includes dissolving a salt. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds). Endothermic Reaction Or Product.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Or Product In endothermic reactions, more energy is. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The absorbed energy provides the activation energy for the reaction to occur. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. These reactions. Endothermic Reaction Or Product.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Or Product The absorbed energy provides the activation energy for the reaction to occur. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. chemical reactions that absorb (or use) energy overall are called endothermic. A good endothermic reaction example includes dissolving a salt. endothermic reactions are chemical reactions in which the. Endothermic Reaction Or Product.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Or Product Investigating endothermic and exothermic reactions aims to estimate energy changes in. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. A good endothermic reaction example includes dissolving a salt. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. in endothermic and exothermic. Endothermic Reaction Or Product.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Endothermic Reaction Or Product endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In endothermic reactions, more energy is. A good endothermic reaction example includes dissolving a salt. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Investigating endothermic and exothermic reactions. Endothermic Reaction Or Product.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Or Product These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. The absorbed energy provides the activation energy for the reaction to occur. In endothermic reactions, more energy is. Investigating endothermic and exothermic reactions aims to estimate. Endothermic Reaction Or Product.
From depositphotos.com
Endothermic Exothermic Reactions Infographic Diagram Showing Relation Endothermic Reaction Or Product an endothermic reaction absorbs heat and cools the environment. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The absorbed energy provides the activation energy for the reaction to occur. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. for an endothermic chemical. Endothermic Reaction Or Product.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Endothermic Reaction Or Product endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. an endothermic reaction is any chemical reaction that absorbs heat from its environment. A good endothermic reaction example includes dissolving a salt.. Endothermic Reaction Or Product.
From wiringdbdementiushl.z4.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction Or Product These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In endothermic reactions, more energy is. Investigating endothermic and exothermic reactions aims to estimate energy changes in. for an endothermic chemical reaction to proceed, the reactants. Endothermic Reaction Or Product.
From drive.cloud.mn
Endothermic Exothermic Reactions Infographic Diagram, 53 OFF Endothermic Reaction Or Product for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. chemical reactions that absorb (or use) energy overall are called endothermic. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Investigating endothermic and exothermic reactions aims to estimate. Endothermic Reaction Or Product.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Or Product A hallmark of this type of reaction is that it feels cold. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. an endothermic reaction absorbs heat and cools the environment. an endothermic. Endothermic Reaction Or Product.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction Or Product The absorbed energy provides the activation energy for the reaction to occur. an endothermic reaction is any chemical reaction that absorbs heat from its environment. an endothermic reaction absorbs heat and cools the environment. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. Investigating endothermic and exothermic reactions aims. Endothermic Reaction Or Product.
From guidelibperplexing.z13.web.core.windows.net
Diagram For Endothermic Reaction Endothermic Reaction Or Product Investigating endothermic and exothermic reactions aims to estimate energy changes in. an endothermic reaction is any chemical reaction that absorbs heat from its environment. an endothermic reaction absorbs heat and cools the environment. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. These reactions lower the temperature. Endothermic Reaction Or Product.
From www.slideserve.com
PPT Enthalpy (H) and Change in Enthalpy ( Δ H ) PowerPoint Endothermic Reaction Or Product These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Investigating endothermic and exothermic reactions aims to estimate energy changes in. The absorbed energy provides the activation energy for the reaction to occur. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. in endothermic and exothermic. Endothermic Reaction Or Product.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Or Product A hallmark of this type of reaction is that it feels cold. an endothermic reaction is any chemical reaction that absorbs heat from its environment. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. In endothermic reactions, more energy is. Investigating endothermic and exothermic reactions aims to estimate. Endothermic Reaction Or Product.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Or Product for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. A hallmark of this type of reaction is that it feels cold. Investigating endothermic and exothermic reactions aims to estimate energy changes in. A good endothermic reaction example includes dissolving a salt. These reactions lower the temperature of their surrounding. Endothermic Reaction Or Product.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Or Product In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. A good endothermic reaction example includes dissolving a salt. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. for an endothermic chemical reaction to proceed, the reactants must absorb energy from. Endothermic Reaction Or Product.
From www.youtube.com
Endothermic and Exothermic Reactions With Potential Energy Diagrams Endothermic Reaction Or Product In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. an endothermic reaction is any chemical reaction that absorbs heat from its environment. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. A hallmark of this type of reaction is. Endothermic Reaction Or Product.
From courses.lumenlearning.com
7.4 Bond Energies and Chemical Reactions The Basics of General Endothermic Reaction Or Product for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In endothermic reactions, more energy is. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds). Endothermic Reaction Or Product.
From www.slideserve.com
PPT & Thermodynamics PowerPoint Presentation, free download Endothermic Reaction Or Product A good endothermic reaction example includes dissolving a salt. chemical reactions that absorb (or use) energy overall are called endothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted. Endothermic Reaction Or Product.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Or Product Investigating endothermic and exothermic reactions aims to estimate energy changes in. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. an endothermic reaction absorbs heat and cools the environment. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. Endothermic Reaction Or Product.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Or Product A hallmark of this type of reaction is that it feels cold. In endothermic reactions, more energy is. The absorbed energy provides the activation energy for the reaction to occur. In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. A good endothermic reaction example includes dissolving a salt. Investigating endothermic and. Endothermic Reaction Or Product.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction Or Product chemical reactions that absorb (or use) energy overall are called endothermic. A hallmark of this type of reaction is that it feels cold. Investigating endothermic and exothermic reactions aims to estimate energy changes in. The absorbed energy provides the activation energy for the reaction to occur. an endothermic reaction is any chemical reaction that absorbs heat from its. Endothermic Reaction Or Product.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Or Product in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. an endothermic reaction is any chemical reaction that absorbs heat from its environment. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. an endothermic reaction absorbs heat and cools the environment. A good. Endothermic Reaction Or Product.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Or Product These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. A hallmark of this type of reaction is that it feels cold. The absorbed energy provides the activation energy for the reaction to occur. chemical reactions that absorb (or use) energy overall are called endothermic. for an endothermic chemical reaction to proceed, the reactants. Endothermic Reaction Or Product.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Endothermic Reaction Or Product A good endothermic reaction example includes dissolving a salt. In endothermic reactions, more energy is. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The absorbed energy provides the activation energy for the reaction to occur. an endothermic reaction is any chemical reaction that absorbs heat from its. Endothermic Reaction Or Product.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Or Product an endothermic reaction is any chemical reaction that absorbs heat from its environment. Investigating endothermic and exothermic reactions aims to estimate energy changes in. A hallmark of this type of reaction is that it feels cold. A good endothermic reaction example includes dissolving a salt. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.. Endothermic Reaction Or Product.