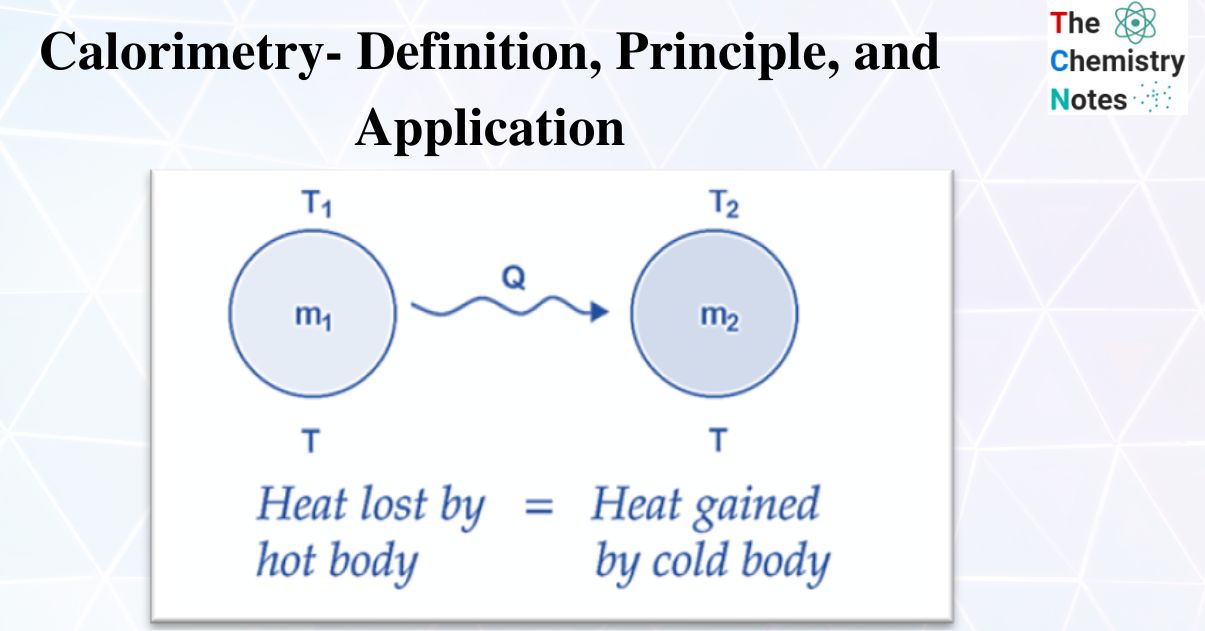
Calorimetry Symbols . q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change.
from thechemistrynotes.com
one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is used to measure. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.
Calorimetry Definition, Principle, Types, Application, and Limitations
Calorimetry Symbols Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
From www.chegg.com
Solved Table 1. Key relationships and definitions for Calorimetry Symbols calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. q = mcδt, where q is the symbol for heat transfer (“quantity of. Calorimetry Symbols.
From www.chegg.com
Solved What do the symbols for all these forumals mean? In Calorimetry Symbols calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The. Calorimetry Symbols.
From www.youtube.com
Numericals of Calorimetry Calorimetry Class 10 Physics ICSE YouTube Calorimetry Symbols calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calorimetry is used to measure. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry is the measurement of the transfer of heat into or out of a system during a. Calorimetry Symbols.
From www.youtube.com
09.05 Calorimetry YouTube Calorimetry Symbols Calorimetry is used to measure. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. one technique we can use to measure. Calorimetry Symbols.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Symbols one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry is the process of measuring the. Calorimetry Symbols.
From www.science-revision.co.uk
Calorimetry Calorimetry Symbols calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. q = mcδt,. Calorimetry Symbols.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Symbols one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry is used to measure the amount. Calorimetry Symbols.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3207019 Calorimetry Symbols calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Calorimetry is used to measure. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process.. Calorimetry Symbols.
From answerzoneguenther.z21.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Symbols q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. one technique we can use to measure the amount of heat involved in a chemical. Calorimetry Symbols.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimetry Symbols one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Calorimetry Symbols.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Symbols calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. one technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry Symbols.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Symbols calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Calorimetry Symbols.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Symbols q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure. Calorimetry Symbols.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Symbols calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the. Calorimetry Symbols.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 Calorimetry Symbols calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure the amount of heat involved in a chemical or physical process. Calorimetry Symbols.
From studylib.net
Heat Equation Calorimetry Symbols q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the measurement of the transfer. Calorimetry Symbols.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimetry Symbols Calorimetry is used to measure. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Calorimetry Symbols.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Symbols calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The symbol c stands for the specific heat (also called “ specific heat. Calorimetry Symbols.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Symbols calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to. Calorimetry Symbols.
From slideplayer.com
Calorimetry Chapter ppt download Calorimetry Symbols calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is used to measure the amount of thermal energy transferred in a. Calorimetry Symbols.
From saylordotorg.github.io
Calorimetry Calorimetry Symbols one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry is the process of measuring the amount of heat released or absorbed during. Calorimetry Symbols.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Symbols q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. one technique we can use to measure the. Calorimetry Symbols.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Symbols one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. one technique we. Calorimetry Symbols.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry Symbols calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. Calorimetry Symbols.
From cmp.utk.edu
Calorimetrychart Center for Materials Processing Calorimetry Symbols q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out. Calorimetry Symbols.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Symbols The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is used to measure the amount of thermal energy transferred in a chemical. Calorimetry Symbols.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Symbols The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. one technique we can. Calorimetry Symbols.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Symbols calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction. Calorimetry Symbols.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Calorimetry Symbols calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. one technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry Symbols.
From chem.libretexts.org
Differential Scanning Calorimetry Chemistry LibreTexts Calorimetry Symbols calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Calorimetry Symbols.
From slideplayer.com
AP Chemistry Zumdahl Notes, 9th ed. ppt download Calorimetry Symbols Calorimetry is used to measure. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Symbols.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5577734 Calorimetry Symbols one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. calorimetry measures enthalpy changes during chemical processes, where. Calorimetry Symbols.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Symbols The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry measures enthalpy changes during chemical processes, where the. Calorimetry Symbols.
From studylib.net
Calorimetry Calorimetry Symbols q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure the amount of thermal energy transferred in a chemical or. Calorimetry Symbols.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Symbols Calorimetry is used to measure. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes,. Calorimetry Symbols.