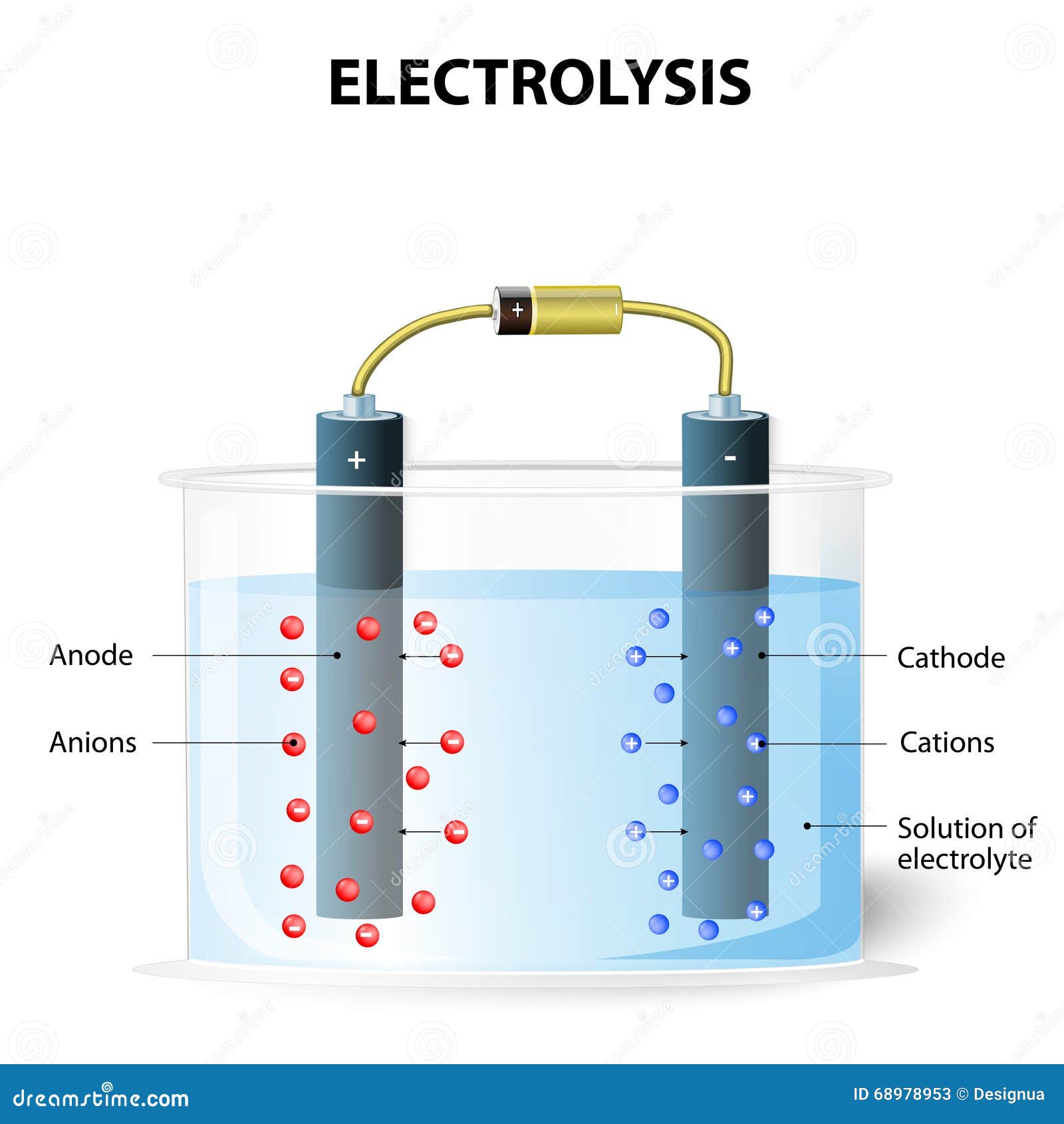
Electrolyte Drawing . Describe variables that influence fluid and electrolyte balance. Name the disorders associated with. Electrolytes are substances that conduct electricity in solution. Electrolytes may be covalent compounds that chemically. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. Substances that dissolve in water to yield ions are called electrolytes. Weak electrolytes, such as hgcl The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. In this experiment, you will use a conductivity tester to determine whether. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. The larger the concentration of ions, the better the solutions conducts. A substance that, when added to water, renders it conductive, is known as an electrolyte. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). A common example of an. By the end of this section, you will be able to:
from www.dreamstime.com
Describe variables that influence fluid and electrolyte balance. The larger the concentration of ions, the better the solutions conducts. List the role of the six most important electrolytes in the body. Electrolytes are substances that conduct electricity in solution. In this experiment, you will use a conductivity tester to determine whether. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). A substance that, when added to water, renders it conductive, is known as an electrolyte. By the end of this section, you will be able to: Substances that dissolve in water to yield ions are called electrolytes. Weak electrolytes, such as hgcl
Electrolysis Stock Illustrations 925 Electrolysis Stock Illustrations
Electrolyte Drawing A common example of an. In this experiment, you will use a conductivity tester to determine whether. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. Electrolytes may be covalent compounds that chemically. Describe variables that influence fluid and electrolyte balance. Electrolytes are substances that conduct electricity in solution. The larger the concentration of ions, the better the solutions conducts. A common example of an. Name the disorders associated with. Weak electrolytes, such as hgcl By the end of this section, you will be able to: An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. Substances that dissolve in water to yield ions are called electrolytes. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. List the role of the six most important electrolytes in the body.
From www.freepik.com
Electrolyte Images Free Vectors, Stock Photos & PSD Electrolyte Drawing A substance that, when added to water, renders it conductive, is known as an electrolyte. The larger the concentration of ions, the better the solutions conducts. In this experiment, you will use a conductivity tester to determine whether. Weak electrolytes, such as hgcl Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry. Electrolyte Drawing.
From www.dreamstime.com
Electrolysis Process Useful for Education in Schools Vector Electrolyte Drawing By the end of this section, you will be able to: Substances that dissolve in water to yield ions are called electrolytes. Describe variables that influence fluid and electrolyte balance. Electrolytes may be covalent compounds that chemically. In this experiment, you will use a conductivity tester to determine whether. The major cations in the body fluid are sodium, potassium, calcium,. Electrolyte Drawing.
From emedsa.org.au
Electrolyte Disorders CoreMed Electrolyte Drawing Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. The larger the concentration of ions, the better the solutions conducts. Electrolytes may be covalent compounds that chemically. Weak electrolytes, such as hgcl A common example of an. Name the disorders associated with. An electrolyte solution conducts electricity because of the. Electrolyte Drawing.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrolyte Drawing The larger the concentration of ions, the better the solutions conducts. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. Describe variables that influence fluid and electrolyte balance. Electrolytes may be covalent compounds that chemically. Name the disorders associated with.. Electrolyte Drawing.
From www.dreamstime.com
Flat Illustration of Electrolysis of Electrolyte Solution in Electrolyte Drawing Electrolytes are substances that conduct electricity in solution. Substances that dissolve in water to yield ions are called electrolytes. By the end of this section, you will be able to: Weak electrolytes, such as hgcl Electrolytes may be covalent compounds that chemically. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative. Electrolyte Drawing.
From www.chemistry4.us
IGCSE ChemistryElectrolysis practice Chemistry For Us Electrolyte Drawing By the end of this section, you will be able to: An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). A common example of an. Electrolytes may be covalent compounds that chemically. Describe variables that influence fluid and electrolyte balance. In this experiment, you will use a conductivity tester to determine whether. The. Electrolyte Drawing.
From www.dreamstime.com
Electroplating with Gold with Auro Cyanide Electrolyte Vector Electrolyte Drawing Describe variables that influence fluid and electrolyte balance. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. The larger the concentration of ions, the better the solutions conducts. Electrolytes may be covalent compounds that chemically. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. The major cations in the. Electrolyte Drawing.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolyte Drawing Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. Electrolytes are substances that conduct electricity in solution. Weak electrolytes, such as hgcl Describe variables that. Electrolyte Drawing.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Electrolyte Drawing A common example of an. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. In this experiment, you will. Electrolyte Drawing.
From www.researchgate.net
Schematic of polymer electrolyte membrane (PEM) methanol electrolyzer Electrolyte Drawing Electrolytes are substances that conduct electricity in solution. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. List the role of the six most important electrolytes in the body. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen. Electrolyte Drawing.
From www.shutterstock.com
19 Strong Electrolyte And Weak Electrolyte Images, Stock Photos Electrolyte Drawing The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Electrolytes are substances that conduct electricity in solution. Name the disorders associated with. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that. Electrolyte Drawing.
From cartoondealer.com
Electrolyte Cartoons, Illustrations & Vector Stock Images 406 Electrolyte Drawing Weak electrolytes, such as hgcl By the end of this section, you will be able to: The larger the concentration of ions, the better the solutions conducts. In this experiment, you will use a conductivity tester to determine whether. The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. Electrolytes may be covalent compounds that. Electrolyte Drawing.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Electrolyte Drawing Electrolytes are substances that conduct electricity in solution. By the end of this section, you will be able to: Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. A substance that, when added to water, renders it conductive, is known as an electrolyte. A common example of an. Describe variables. Electrolyte Drawing.
From cartoondealer.com
Anode And Cathode Scientific Physics Education Diagram, Vector Electrolyte Drawing The major anions are chloride, bicarbonate, sulfate, and proteinate ions. Electrolytes may be covalent compounds that chemically. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. Describe variables that influence fluid and electrolyte balance. The larger the concentration of ions, the better the solutions conducts. List the role of the. Electrolyte Drawing.
From ourfuture.energy
Light from salt OurFuture.Energy Electrolyte Drawing Electrolytes are substances that conduct electricity in solution. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Electrolytes may be covalent compounds that chemically. By the end of this section, you will be able to: Substances that dissolve in water to yield ions are called electrolytes. The major anions are chloride, bicarbonate, sulfate,. Electrolyte Drawing.
From clipart-library.com
Free Electrolyte Cliparts, Download Free Electrolyte Cliparts png Electrolyte Drawing Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. Electrolytes are substances that conduct electricity in solution. The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. By the end of this section, you will be able to: The major anions are chloride, bicarbonate, sulfate,. Electrolyte Drawing.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrolyte Drawing List the role of the six most important electrolytes in the body. A common example of an. In this experiment, you will use a conductivity tester to determine whether. Electrolytes may be covalent compounds that chemically. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. The major anions are chloride,. Electrolyte Drawing.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Electrolyte Drawing Electrolytes are substances that conduct electricity in solution. The larger the concentration of ions, the better the solutions conducts. A common example of an. A substance that, when added to water, renders it conductive, is known as an electrolyte. Describe variables that influence fluid and electrolyte balance. An electrolyte solution conducts electricity because of the movement of ions in the. Electrolyte Drawing.
From www.osmosis.org
Overview of Electrolyte Balance Osmosis Video Library Electrolyte Drawing List the role of the six most important electrolytes in the body. Electrolytes may be covalent compounds that chemically. Substances that dissolve in water to yield ions are called electrolytes. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. Weak electrolytes, such as hgcl A common example of an. The major cations in the body fluid are sodium, potassium,. Electrolyte Drawing.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Electrolyte Drawing A substance that, when added to water, renders it conductive, is known as an electrolyte. Substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically. Electrolytes are substances that conduct electricity in solution. By the end of this section, you will be able to: Name the disorders associated with. The major anions. Electrolyte Drawing.
From www.animalia-life.club
Electrolyte Chemistry Electrolyte Drawing List the role of the six most important electrolytes in the body. Name the disorders associated with. The larger the concentration of ions, the better the solutions conducts. In this experiment, you will use a conductivity tester to determine whether. By the end of this section, you will be able to: Substances that dissolve in water to yield ions are. Electrolyte Drawing.
From www.istockphoto.com
Electrolyte Illustrations, RoyaltyFree Vector Graphics & Clip Art iStock Electrolyte Drawing Electrolytes are substances that conduct electricity in solution. Name the disorders associated with. Electrolytes may be covalent compounds that chemically. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. Describe variables that influence fluid and electrolyte balance. By the end of this section, you will be able to: A substance. Electrolyte Drawing.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolyte Drawing The major anions are chloride, bicarbonate, sulfate, and proteinate ions. Electrolytes may be covalent compounds that chemically. Weak electrolytes, such as hgcl Describe variables that influence fluid and electrolyte balance. Substances that dissolve in water to yield ions are called electrolytes. List the role of the six most important electrolytes in the body. An electrolyte solution conducts electricity because of. Electrolyte Drawing.
From www.dreamstime.com
Electrolytic Cell Infographic Diagram with Components Stock Vector Electrolyte Drawing Describe variables that influence fluid and electrolyte balance. A common example of an. Substances that dissolve in water to yield ions are called electrolytes. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). By. Electrolyte Drawing.
From www.majordifferences.com
Strong Electrolytes vs Weak Electrolytes Major Differences Electrolyte Drawing Weak electrolytes, such as hgcl Substances that dissolve in water to yield ions are called electrolytes. By the end of this section, you will be able to: A common example of an. Describe variables that influence fluid and electrolyte balance. Electrolytes may be covalent compounds that chemically. Electrolytes are substances that conduct electricity in solution. The major cations in the. Electrolyte Drawing.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Electrolyte Drawing The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. A common example of an. Describe variables that influence fluid and electrolyte balance. In this experiment, you will use a conductivity tester to determine whether. Name the disorders associated with. Substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent. Electrolyte Drawing.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Electrolyte Drawing List the role of the six most important electrolytes in the body. Electrolytes are substances that conduct electricity in solution. Substances that dissolve in water to yield ions are called electrolytes. A common example of an. Name the disorders associated with. A substance that, when added to water, renders it conductive, is known as an electrolyte. The larger the concentration. Electrolyte Drawing.
From www.osmosis.org
Fluid and Electrolyte Balance Osmosis Video Library Electrolyte Drawing Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. A common example of an. A substance that, when added to water, renders it conductive, is known as an electrolyte. Substances that dissolve in water to yield ions are called electrolytes. The major cations in the body fluid are sodium, potassium,. Electrolyte Drawing.
From www.dreamstime.com
Electrolysis Stock Illustrations 925 Electrolysis Stock Illustrations Electrolyte Drawing An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Electrolytes are substances that conduct electricity in solution. By the end of this section, you will be able to: The major anions are chloride, bicarbonate, sulfate, and proteinate ions. The larger the concentration of ions, the better the solutions conducts. Substances that dissolve in. Electrolyte Drawing.
From www.vrogue.co
Strong Electrolyte Definition And Examples vrogue.co Electrolyte Drawing Weak electrolytes, such as hgcl The larger the concentration of ions, the better the solutions conducts. Substances that dissolve in water to yield ions are called electrolytes. A common example of an. Describe variables that influence fluid and electrolyte balance. A substance that, when added to water, renders it conductive, is known as an electrolyte. Electrolytes may be covalent compounds. Electrolyte Drawing.
From www.shutterstock.com
19 Strong Electrolyte And Weak Electrolyte Images, Stock Photos Electrolyte Drawing Electrolytes may be covalent compounds that chemically. Electrolytes are substances that conduct electricity in solution. By the end of this section, you will be able to: A common example of an. In this experiment, you will use a conductivity tester to determine whether. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The. Electrolyte Drawing.
From www.bioticsresearch.com
ElectrolyteForte Electrolyte Drawing By the end of this section, you will be able to: Name the disorders associated with. Electrolytes are substances that conduct electricity in solution. In this experiment, you will use a conductivity tester to determine whether. Weak electrolytes, such as hgcl List the role of the six most important electrolytes in the body. A substance that, when added to water,. Electrolyte Drawing.
From healthjade.com
Electrolytes Sources and Foods High In Electrolytes Electrolyte Drawing In this experiment, you will use a conductivity tester to determine whether. The larger the concentration of ions, the better the solutions conducts. A substance that, when added to water, renders it conductive, is known as an electrolyte. A common example of an. The major anions are chloride, bicarbonate, sulfate, and proteinate ions. An electrolyte solution conducts electricity because of. Electrolyte Drawing.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Electrolyte Drawing A common example of an. Electrolytes may be covalent compounds that chemically. The major cations in the body fluid are sodium, potassium, calcium, magnesium, and hydrogen ions. Electrolytes in body fluids are active chemicals or cations that carry positive charges and anions that carry negative charges. A substance that, when added to water, renders it conductive, is known as an. Electrolyte Drawing.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolyte Drawing By the end of this section, you will be able to: Electrolytes are substances that conduct electricity in solution. In this experiment, you will use a conductivity tester to determine whether. Substances that dissolve in water to yield ions are called electrolytes. Weak electrolytes, such as hgcl A substance that, when added to water, renders it conductive, is known as. Electrolyte Drawing.