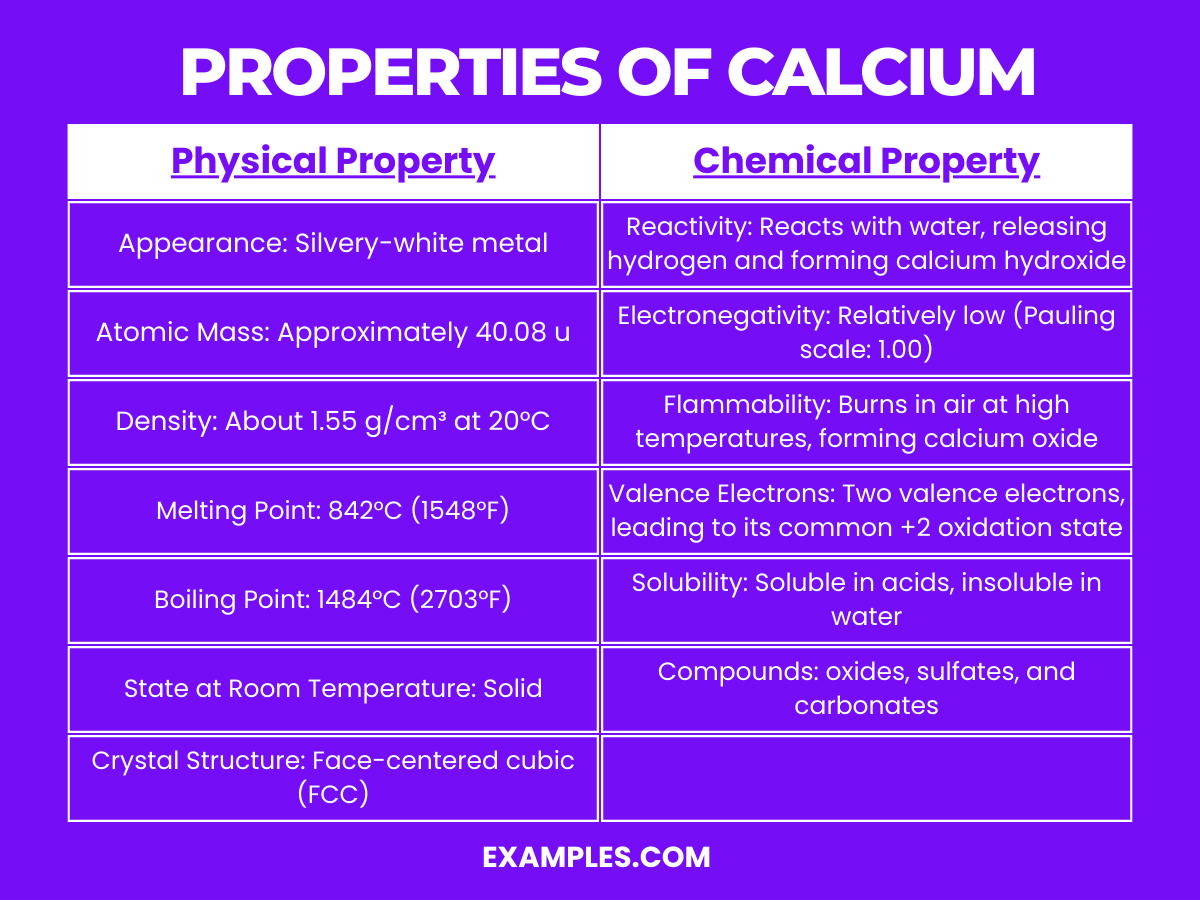
What Are The Properties Of Calcium Chloride . Calcium chloride is an ionic compound with the chemical formula cacl2. It is a crystalline solid white in colour at room. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Calcium chloride is an ionic compound composed of chlorine and calcium. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. The density of calcium chloride is 2.15 gm/cm 3. Calcium chloride has a crystalline. It is soluble both in inorganic solvents like water, as well as. Therefore, a sample of calcium chloride. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Calcium chloride is shipped in three basic forms: Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn.
from www.examples.com
(1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Calcium chloride has a crystalline. Calcium chloride is an ionic compound composed of chlorine and calcium. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. Calcium chloride is an ionic compound with the chemical formula cacl2. The density of calcium chloride is 2.15 gm/cm 3. Therefore, a sample of calcium chloride. It is soluble both in inorganic solvents like water, as well as. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions.
Calcium (Ca) Preparation, Properties, Uses, Compounds, Reactivity
What Are The Properties Of Calcium Chloride In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. It is soluble both in inorganic solvents like water, as well as. The density of calcium chloride is 2.15 gm/cm 3. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Calcium chloride is an ionic compound composed of chlorine and calcium. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Calcium chloride is an ionic compound with the chemical formula cacl2. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Calcium chloride is shipped in three basic forms: It is a crystalline solid white in colour at room. Calcium chloride has a crystalline. Therefore, a sample of calcium chloride.
From www.ytzbsm.com
China Calcium Chloride Pellets Manufacturers and Factory, Suppliers What Are The Properties Of Calcium Chloride Calcium chloride is an ionic compound composed of chlorine and calcium. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. Calcium chloride is an ionic compound with the chemical formula cacl2. The density. What Are The Properties Of Calcium Chloride.
From foodcom.pl
Calcium Chloride properties and common uses What Are The Properties Of Calcium Chloride Calcium chloride is an ionic compound composed of chlorine and calcium. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. It is a crystalline solid white in colour at room. The density of calcium chloride is 2.15 gm/cm 3. Calcium chloride is shipped in three basic forms: Cacl. What Are The Properties Of Calcium Chloride.
From www.dchemie.com.my
Calcium Chloride, Food Grade DChemie Malaysia What Are The Properties Of Calcium Chloride Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Calcium chloride is an ionic compound composed of chlorine and calcium. Calcium chloride has a crystalline. Therefore, a sample of calcium chloride. Calcium chloride is shipped in three basic forms: (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized. What Are The Properties Of Calcium Chloride.
From medimpextrade.com
What is Calcium Chloride? Properties and uses MedimpexTrade What Are The Properties Of Calcium Chloride Therefore, a sample of calcium chloride. The density of calcium chloride is 2.15 gm/cm 3. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. It is a crystalline solid white in colour at room. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing. What Are The Properties Of Calcium Chloride.
From proper-cooking.info
Structure Of Calcium Chloride What Are The Properties Of Calcium Chloride Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Therefore, a sample of calcium chloride. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a. What Are The Properties Of Calcium Chloride.
From addisonabbhendricks.blogspot.com
Chemical Properties of Calcium Chloride AddisonabbHendricks What Are The Properties Of Calcium Chloride Calcium chloride is an ionic compound composed of chlorine and calcium. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Therefore, a sample of calcium chloride. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Cacl 2 is highly water soluble, hygroscopic (absorbs. What Are The Properties Of Calcium Chloride.
From www.youtube.com
calcium chloride uses Price कैल्शियम क्लोराइड YouTube What Are The Properties Of Calcium Chloride Therefore, a sample of calcium chloride. It is a crystalline solid white in colour at room. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. It is soluble both in inorganic solvents like water, as well. What Are The Properties Of Calcium Chloride.
From edurev.in
Calcium is obtained by thea)Roasting of limestoneb)Electrolysis of a What Are The Properties Of Calcium Chloride Calcium chloride is shipped in three basic forms: Calcium chloride is an ionic compound with the chemical formula cacl2. It is soluble both in inorganic solvents like water, as well as. Therefore, a sample of calcium chloride. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Calcium chloride is an ionic. What Are The Properties Of Calcium Chloride.
From www.procurementresource.com
Calcium Chloride Production Cost Analysis Reports 2024 What Are The Properties Of Calcium Chloride The density of calcium chloride is 2.15 gm/cm 3. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Calcium chloride has a crystalline. It is soluble both in inorganic solvents like water, as well as. Calcium chloride is shipped in three basic forms: Calcium chloride is an ionic compound with the chemical formula cacl2.. What Are The Properties Of Calcium Chloride.
From www.ebay.co.uk
Calcium Chloride, Deicing agent, white flakes, 100g25kg eBay What Are The Properties Of Calcium Chloride The density of calcium chloride is 2.15 gm/cm 3. It is soluble both in inorganic solvents like water, as well as. It is a crystalline solid white in colour at room. Calcium chloride is shipped in three basic forms: Calcium chloride is an ionic compound composed of chlorine and calcium. (1) flake, pebble and powdered form containing 77 to 80%. What Are The Properties Of Calcium Chloride.
From www.tradeindia.com
Calcium Chloride at Best Price in Ahmedabad, Gujarat Kalpana What Are The Properties Of Calcium Chloride Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. It is soluble both in inorganic solvents like water, as well as. Calcium chloride is shipped in three basic forms: Calcium chloride has a crystalline. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Therefore, a. What Are The Properties Of Calcium Chloride.
From shanghaichemex.com
Calcium chloride Shanghai Chemex Group Ltd. What Are The Properties Of Calcium Chloride Calcium chloride is an ionic compound composed of chlorine and calcium. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. Its density is 2.15 g/cm³, and it is. What Are The Properties Of Calcium Chloride.
From revolutionfermentation.com
What Is Calcium Chloride And How to Use It? Revolution Fermentation What Are The Properties Of Calcium Chloride It is a crystalline solid white in colour at room. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. Calcium chloride is an ionic compound with the chemical formula cacl2. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn.. What Are The Properties Of Calcium Chloride.
From chemicaldb.netlify.app
What is used to make calcium chloride What Are The Properties Of Calcium Chloride Therefore, a sample of calcium chloride. It is soluble both in inorganic solvents like water, as well as. Calcium chloride has a crystalline. Calcium chloride is shipped in three basic forms: Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Calcium chloride is an ionic compound composed of chlorine and calcium.. What Are The Properties Of Calcium Chloride.
From www.britannica.com
Calcium Definition, Properties, & Compounds Britannica What Are The Properties Of Calcium Chloride Calcium chloride is an ionic compound with the chemical formula cacl2. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. The density of calcium chloride is 2.15 gm/cm 3. Calcium chloride is an ionic compound composed of chlorine and calcium. Therefore, a sample of calcium chloride. It is soluble. What Are The Properties Of Calcium Chloride.
From medium.com
Unleashing the Power of Calcium Chloride A Versatile Chemical Marvel What Are The Properties Of Calcium Chloride It is soluble both in inorganic solvents like water, as well as. It is a crystalline solid white in colour at room. The density of calcium chloride is 2.15 gm/cm 3. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. In terms of its chemical properties, calcium chloride is. What Are The Properties Of Calcium Chloride.
From www.youtube.com
Calcium chloride YouTube What Are The Properties Of Calcium Chloride Calcium chloride is an ionic compound composed of chlorine and calcium. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Calcium chloride is shipped in three basic forms: It is a crystalline solid white. What Are The Properties Of Calcium Chloride.
From absortech.com
What is calcium chloride and why do we use it? Absortech What Are The Properties Of Calcium Chloride (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Calcium chloride is shipped in three basic forms: In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. It is soluble both in inorganic solvents like water, as well. What Are The Properties Of Calcium Chloride.
From brainly.ph
To prepare a 1L of 1000ppm solution of calcium chloride, how many grams What Are The Properties Of Calcium Chloride (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. It is soluble both in inorganic solvents like water, as well as. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Calcium chloride is shipped in three basic forms: Calcium chloride is an ionic. What Are The Properties Of Calcium Chloride.
From formulasense.com
What is Calcium Chloride? — Formula Sense What Are The Properties Of Calcium Chloride The density of calcium chloride is 2.15 gm/cm 3. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Calcium chloride is shipped in three basic forms: Calcium chloride has a crystalline. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%.. What Are The Properties Of Calcium Chloride.
From www.askdifference.com
Calcium Chloride vs. Calcium Chloride Dihydrate — What’s the Difference? What Are The Properties Of Calcium Chloride Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Therefore, a sample of calcium chloride. Calcium chloride is shipped in three basic forms: The density of calcium chloride is 2.15 gm/cm 3. It is soluble both in inorganic solvents like water, as well as. It is a crystalline solid white in colour at room.. What Are The Properties Of Calcium Chloride.
From www.aliexpress.com
200g Of Calcium Chloride E509 Molecular Cuisine Cheese Making What Are The Properties Of Calcium Chloride Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Calcium chloride is an ionic compound with the chemical formula cacl2. Therefore, a sample of calcium chloride. The density of calcium chloride is 2.15 gm/cm 3. Calcium chloride has a crystalline. Its density is 2.15 g/cm³, and it is highly soluble in. What Are The Properties Of Calcium Chloride.
From marketmirrorwire.blogspot.com
Calcium chloride Market Size to hit US 1.7 Billion by 2028 Report by What Are The Properties Of Calcium Chloride (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Calcium chloride is an ionic compound composed of chlorine and calcium. Calcium chloride is shipped in three basic forms: Calcium chloride is an ionic compound with the chemical formula cacl2. Calcium chloride has a crystalline. Therefore, a sample of calcium. What Are The Properties Of Calcium Chloride.
From camachem.com
Calcium Chloride 74 Flakes What Are The Properties Of Calcium Chloride It is a crystalline solid white in colour at room. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. The density of calcium chloride is 2.15 gm/cm 3. It is soluble. What Are The Properties Of Calcium Chloride.
From www.slideserve.com
PPT Calcium Chloride PowerPoint Presentation, free download ID2156417 What Are The Properties Of Calcium Chloride The density of calcium chloride is 2.15 gm/cm 3. Therefore, a sample of calcium chloride. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. Calcium chloride is shipped in three basic. What Are The Properties Of Calcium Chloride.
From www.differencebetween.com
Difference Between Calcium Chloride and Sodium Chloride Compare the What Are The Properties Of Calcium Chloride The density of calcium chloride is 2.15 gm/cm 3. Calcium chloride has a crystalline. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. Calcium chloride is shipped in three basic forms: Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. (1) flake, pebble and powdered. What Are The Properties Of Calcium Chloride.
From foodcom.pl
Calcium Chloride (E509) Price 25 kg, Big Bags B2B Trade What Are The Properties Of Calcium Chloride Calcium chloride is shipped in three basic forms: The density of calcium chloride is 2.15 gm/cm 3. Calcium chloride has a crystalline. It is a crystalline solid white in colour at room. It is soluble both in inorganic solvents like water, as well as. Calcium chloride is an ionic compound composed of chlorine and calcium. Therefore, a sample of calcium. What Are The Properties Of Calcium Chloride.
From testbook.com
Calcium chloride formula Structure, Molecular Formula & Uses What Are The Properties Of Calcium Chloride Calcium chloride is an ionic compound composed of chlorine and calcium. Calcium chloride is shipped in three basic forms: Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. It is a crystalline solid white in colour at room. In terms of its chemical properties, calcium chloride is highly soluble in water. What Are The Properties Of Calcium Chloride.
From chemicaldb.netlify.app
What does calcium chloride smell like What Are The Properties Of Calcium Chloride Therefore, a sample of calcium chloride. Calcium chloride is an ionic compound composed of chlorine and calcium. Calcium chloride has a crystalline. It is a crystalline solid white in colour at room. Calcium chloride is shipped in three basic forms: (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%.. What Are The Properties Of Calcium Chloride.
From www.indiamart.com
Calcium Chloride Anhydrous, Grade Standard Chemical Grade, Packaging What Are The Properties Of Calcium Chloride In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. Calcium chloride has a crystalline. Cacl 2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn. It is a crystalline solid white in colour at room. It is soluble both in inorganic. What Are The Properties Of Calcium Chloride.
From flashugnews.com
How Does Calcium Chloride Dihydrate Differ From Calcium Chloride What Are The Properties Of Calcium Chloride Therefore, a sample of calcium chloride. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Calcium chloride is shipped in three basic forms: Calcium chloride is an ionic compound composed of chlorine and calcium. Calcium chloride is an ionic compound with the chemical formula cacl2. Calcium chloride has a. What Are The Properties Of Calcium Chloride.
From www.di-corp.com
Calcium Chloride 9497 Mini Prills Get a Quote Now What Are The Properties Of Calcium Chloride Calcium chloride is shipped in three basic forms: It is a crystalline solid white in colour at room. Calcium chloride is an ionic compound with the chemical formula cacl2. The density of calcium chloride is 2.15 gm/cm 3. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. It. What Are The Properties Of Calcium Chloride.
From www.examples.com
Calcium (Ca) Preparation, Properties, Uses, Compounds, Reactivity What Are The Properties Of Calcium Chloride Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. Calcium chloride is an ionic compound with the chemical formula cacl2. Therefore, a sample of calcium chloride. It is soluble both in inorganic solvents. What Are The Properties Of Calcium Chloride.
From www.petroacc.com
Calcium Chloride Product of Aras Petrochemical Company What Are The Properties Of Calcium Chloride It is a crystalline solid white in colour at room. It is soluble both in inorganic solvents like water, as well as. The density of calcium chloride is 2.15 gm/cm 3. (1) flake, pebble and powdered form containing 77 to 80% calcium chloride, (2) solid, crystallized form containing 73 to 75%. Calcium chloride is shipped in three basic forms: Therefore,. What Are The Properties Of Calcium Chloride.
From revolutionfermentation.com
What Is Calcium Chloride And How to Use It? Revolution Fermentation What Are The Properties Of Calcium Chloride In terms of its chemical properties, calcium chloride is highly soluble in water and releases a significant amount of heat upon. Its density is 2.15 g/cm³, and it is highly soluble in water, forming colorless solutions. The density of calcium chloride is 2.15 gm/cm 3. Calcium chloride has a crystalline. (1) flake, pebble and powdered form containing 77 to 80%. What Are The Properties Of Calcium Chloride.