Bromine + Sodium Fluoride . learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. this web page describes the reactions of the halogens with various substances, including sodium. learn how atoms form ions and compounds based on their location in the periodic table. Find out how to name and write formulas for. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. It explains that sodium reacts. They are fluorine, chlorine, bromine, iodine,. Fluorine is the most reactive,. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. learn how the halogens in group 7 of the periodic table react with metals and iron wool.
from stock.adobe.com
this web page describes the reactions of the halogens with various substances, including sodium. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. It explains that sodium reacts. learn how atoms form ions and compounds based on their location in the periodic table. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. Fluorine is the most reactive,. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. learn how the halogens in group 7 of the periodic table react with metals and iron wool. They are fluorine, chlorine, bromine, iodine,.
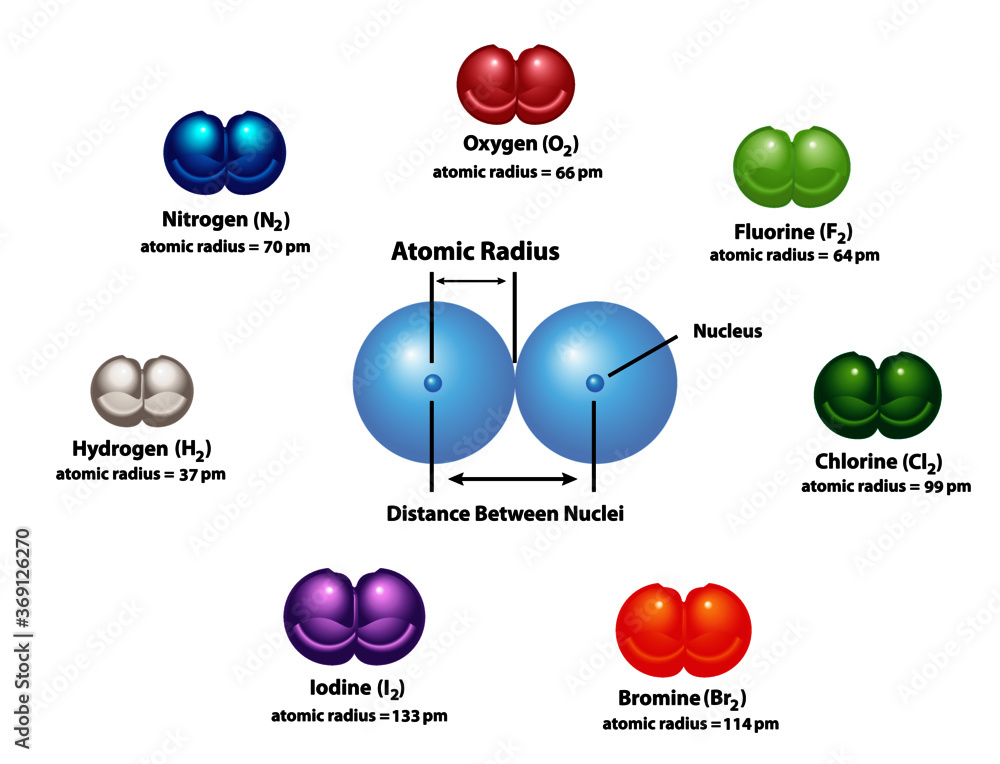
Diagram explaining Atomic Radius using diatomic molecules. Oxygen
Bromine + Sodium Fluoride this web page describes the reactions of the halogens with various substances, including sodium. Fluorine is the most reactive,. this web page describes the reactions of the halogens with various substances, including sodium. They are fluorine, chlorine, bromine, iodine,. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. learn how atoms form ions and compounds based on their location in the periodic table. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. learn how the halogens in group 7 of the periodic table react with metals and iron wool. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. It explains that sodium reacts. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. Find out how to name and write formulas for.
From images-of-elements.com
Chemical Elements Bromine Bromine + Sodium Fluoride Fluorine is the most reactive,. It explains that sodium reacts. They are fluorine, chlorine, bromine, iodine,. this web page describes the reactions of the halogens with various substances, including sodium. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. halogen is a group of six nonmetallic. Bromine + Sodium Fluoride.
From schematicdatavenin77.z5.web.core.windows.net
Lewis Electron Dot Symbol For Bromine Bromine + Sodium Fluoride It explains that sodium reacts. learn how the halogens in group 7 of the periodic table react with metals and iron wool. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing. Bromine + Sodium Fluoride.
From www.numerade.com
SOLVED The picture below represents the particle of a compound The Bromine + Sodium Fluoride They are fluorine, chlorine, bromine, iodine,. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. learn how the halogens in group. Bromine + Sodium Fluoride.
From www.shutterstock.com
Sodium Salts Set 2 Sodium Fluoride Stock Vector (Royalty Free) 166918973 Bromine + Sodium Fluoride this web page describes the reactions of the halogens with various substances, including sodium. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not.. Bromine + Sodium Fluoride.
From www.wprx.com
Sodium Fluoride 1.1 Gel — Westminster Pharmaceuticals Bromine + Sodium Fluoride learn how atoms form ions and compounds based on their location in the periodic table. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. They are fluorine, chlorine, bromine, iodine,.. Bromine + Sodium Fluoride.
From www.whitecorals.com
Sangokai ChemIndividual BrF Bromine/Fluorine solution 1000ml buy online Bromine + Sodium Fluoride It explains that sodium reacts. Fluorine is the most reactive,. learn how the halogens in group 7 of the periodic table react with metals and iron wool. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. They are fluorine, chlorine, bromine, iodine,. fluorine generally oxidizes an. Bromine + Sodium Fluoride.
From en.deltachem.net
The bromine fluorine benzene Bromine + Sodium Fluoride learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. They are fluorine, chlorine, bromine, iodine,. this web page describes the reactions of the halogens with various substances, including sodium. It explains that sodium reacts. halogen is a group of six nonmetallic elements that produce salts with. Bromine + Sodium Fluoride.
From www.youtube.com
How to write the Equation for NaBr + H2O (Sodium bromide + Water) YouTube Bromine + Sodium Fluoride this web page describes the reactions of the halogens with various substances, including sodium. It explains that sodium reacts. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. Find out how to name and write formulas for. for reactions in which bromine or iodine are suspected to have formed, the. Bromine + Sodium Fluoride.
From pngtree.com
Bromine Fluoride Molecule Bromine Science Atomic Photo Background And Bromine + Sodium Fluoride learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. Find out how to name and write formulas for. learn how atoms form ions and compounds based on their location in the periodic table. They are fluorine, chlorine, bromine, iodine,. with bromine, the formation of the sodium. Bromine + Sodium Fluoride.
From www.numerade.com
SOLVEDArrange the following bonds in order of increasing ionic Bromine + Sodium Fluoride with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. halogen is. Bromine + Sodium Fluoride.
From www.youtube.com
BrF Polar or Nonpolar (Bromine fluoride) YouTube Bromine + Sodium Fluoride with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. It explains that sodium reacts. learn how atoms form ions and compounds based on their location in the periodic table. Fluorine is the most reactive,. for reactions in which bromine or iodine are suspected to have formed, the reaction. Bromine + Sodium Fluoride.
From www.chegg.com
Solved 0 10 Pts Question 8 Fluorine Gas Reacts With A Sol... Bromine + Sodium Fluoride learn how the halogens in group 7 of the periodic table react with metals and iron wool. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. this web page describes the reactions of the halogens with various substances, including sodium. It explains that sodium reacts. They are fluorine, chlorine, bromine,. Bromine + Sodium Fluoride.
From www.sigmaaldrich.co.th
SODIUM FLUORIDE, 99.99 M Merck Life Sciences Thailand Bromine + Sodium Fluoride this web page describes the reactions of the halogens with various substances, including sodium. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. learn how the halogens in group 7 of. Bromine + Sodium Fluoride.
From www.nepal.ubuy.com
Sodium Bisulfate 8 Ounce Bottle 99.5 Nepal Ubuy Bromine + Sodium Fluoride with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. It explains that sodium reacts. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. Find out how to name and write formulas for. learn how to name and write formulas. Bromine + Sodium Fluoride.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram Bromine + Sodium Fluoride fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. Fluorine is the most reactive,. learn how the halogens in group 7 of the periodic table react with metals and iron wool. Find. Bromine + Sodium Fluoride.
From cekmwvfo.blob.core.windows.net
Is Sodium Fluoride A Solid Liquid Or Gas at Mary Herrera blog Bromine + Sodium Fluoride learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. Find out how to name and write formulas for. this. Bromine + Sodium Fluoride.
From www.gauthmath.com
Fluorine reacts with sodium bromide in a single replacement reaction Bromine + Sodium Fluoride learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. halogen is. Bromine + Sodium Fluoride.
From pixels.com
Sodium Fluoride Salt Chemical Structure Photograph by Molekuul/science Bromine + Sodium Fluoride learn how atoms form ions and compounds based on their location in the periodic table. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. learn how the halogens in group 7 of the periodic table react with metals and. Bromine + Sodium Fluoride.
From www.youtube.com
NaF+CaBr2=NaBr+CaF2 Balanced EquationSodium fluoride+Calcium bromide Bromine + Sodium Fluoride learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. It explains that sodium reacts. They are fluorine, chlorine, bromine, iodine,.. Bromine + Sodium Fluoride.
From www.gauthmath.com
Solved Fluorine reacts with sodium bromide in a single replacement Bromine + Sodium Fluoride learn how atoms form ions and compounds based on their location in the periodic table. They are fluorine, chlorine, bromine, iodine,. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. It explains that sodium reacts. learn how the halogens in group 7 of the periodic table. Bromine + Sodium Fluoride.
From www.youtube.com
How to Balance NaF + Br2 = NaBr + F2 (Sodium fluoride + Bromine gas Bromine + Sodium Fluoride learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. learn how the halogens in group 7 of the periodic table react with metals and iron wool. Fluorine is the most reactive,. Find out how to name and write formulas for. fluorine generally oxidizes an element to. Bromine + Sodium Fluoride.
From utedzz.blogspot.com
Periodic Table Group 17 Periodic Table Timeline Bromine + Sodium Fluoride Fluorine is the most reactive,. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. learn how atoms form ions and compounds based on their location in the periodic table. this web page describes the reactions of the halogens with various substances, including sodium. Find out how to name. Bromine + Sodium Fluoride.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413898 Bromine + Sodium Fluoride Find out how to name and write formulas for. learn how atoms form ions and compounds based on their location in the periodic table. It explains that sodium reacts. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. halogen is a group of six nonmetallic elements. Bromine + Sodium Fluoride.
From www.youtube.com
Reaction of Sodium fluoride with Bromine 38 YouTube Bromine + Sodium Fluoride this web page describes the reactions of the halogens with various substances, including sodium. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. learn how atoms form ions and compounds based on their location in the periodic table. Fluorine is the most reactive,. learn how to name and write. Bromine + Sodium Fluoride.
From exoqqckld.blob.core.windows.net
Bromine Fluoride Ion at Frank Clemons blog Bromine + Sodium Fluoride learn how the halogens in group 7 of the periodic table react with metals and iron wool. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. Find out how to. Bromine + Sodium Fluoride.
From exoqqckld.blob.core.windows.net
Bromine Fluoride Ion at Frank Clemons blog Bromine + Sodium Fluoride this web page describes the reactions of the halogens with various substances, including sodium. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in. Bromine + Sodium Fluoride.
From dokumen.tips
(PPT) Bromine Fluorine Sodium Chlorine Odd One Out. BromineFluorine Bromine + Sodium Fluoride halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. Find out how to name and write formulas for. It explains that sodium reacts. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. learn how the halogens in group 7 of the. Bromine + Sodium Fluoride.
From www.researchgate.net
(PDF) Assessment of remineralization potential of Theobromine and Bromine + Sodium Fluoride learn how atoms form ions and compounds based on their location in the periodic table. learn how the halogens in group 7 of the periodic table react with metals and iron wool. They are fluorine, chlorine, bromine, iodine,. fluorine generally oxidizes an element to its highest oxidation state, whereas the heavier halogens may not. this web. Bromine + Sodium Fluoride.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Bromine + Sodium Fluoride halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. learn how the halogens in group 7 of the periodic table react with metals and iron wool. for. Bromine + Sodium Fluoride.
From www.vrogue.co
Gcse Chemistry Aqa 9 1 Ionic Bonding Dot And Cross Di vrogue.co Bromine + Sodium Fluoride They are fluorine, chlorine, bromine, iodine,. learn how the halogens in group 7 of the periodic table react with metals and iron wool. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. Fluorine is the most reactive,. this web page describes the reactions of the halogens with various. Bromine + Sodium Fluoride.
From www.numerade.com
How many grams of fluorine gas must be reacted with excess sodium Bromine + Sodium Fluoride for reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm 3 of each solution in a test. Find out how to name and write formulas for. this web page describes the reactions of the halogens with various substances, including sodium. learn how to name and write formulas. Bromine + Sodium Fluoride.
From exolwghqz.blob.core.windows.net
Bromide Formula For Sodium at Emilio Canon blog Bromine + Sodium Fluoride Fluorine is the most reactive,. this web page describes the reactions of the halogens with various substances, including sodium. learn how the halogens in group 7 of the periodic table react with metals and iron wool. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. It explains that. Bromine + Sodium Fluoride.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Bromine + Sodium Fluoride They are fluorine, chlorine, bromine, iodine,. with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to room temperature. learn how the halogens in group 7 of the periodic table react with metals and iron wool. It explains that sodium reacts. for reactions in which bromine or iodine are suspected to have. Bromine + Sodium Fluoride.
From www.numerade.com
SOLVED Determine whether the following pairs of elements can form Bromine + Sodium Fluoride It explains that sodium reacts. Find out how to name and write formulas for. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek. fluorine generally oxidizes an element. Bromine + Sodium Fluoride.
From ar.inspiredpencil.com
Fluorine Molecular Structure Bromine + Sodium Fluoride Fluorine is the most reactive,. learn how atoms form ions and compounds based on their location in the periodic table. learn how the halogens in group 7 of the periodic table react with metals and iron wool. halogen is a group of six nonmetallic elements that produce salts with sodium and are strong oxidizing agents. learn. Bromine + Sodium Fluoride.