Cooler Energy Definition . the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc An object cannot contain heat, but it can lose or gain energy in the form. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. Describe the physical meaning of temperature. define heat and work, and describe an important limitation in their interconversion. Atoms and molecules inherently have kinetic and thermal. Can transfer by heating from a hotter region to a cooler region. energy is a conserved quantity. The temperature of the hotter. Skip to main content if you're seeing this message, it means we're. heat transfer occurs when thermal energy moves from one place to another. in science, “heat” is the transfer of energy from a warmer object to a cooler one. learn what thermal energy is and how to calculate it.
from gehde.com.au
in science, “heat” is the transfer of energy from a warmer object to a cooler one. energy is a conserved quantity. Skip to main content if you're seeing this message, it means we're. Atoms and molecules inherently have kinetic and thermal. heat transfer occurs when thermal energy moves from one place to another. Can transfer by heating from a hotter region to a cooler region. define heat and work, and describe an important limitation in their interconversion. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. learn what thermal energy is and how to calculate it. The temperature of the hotter.
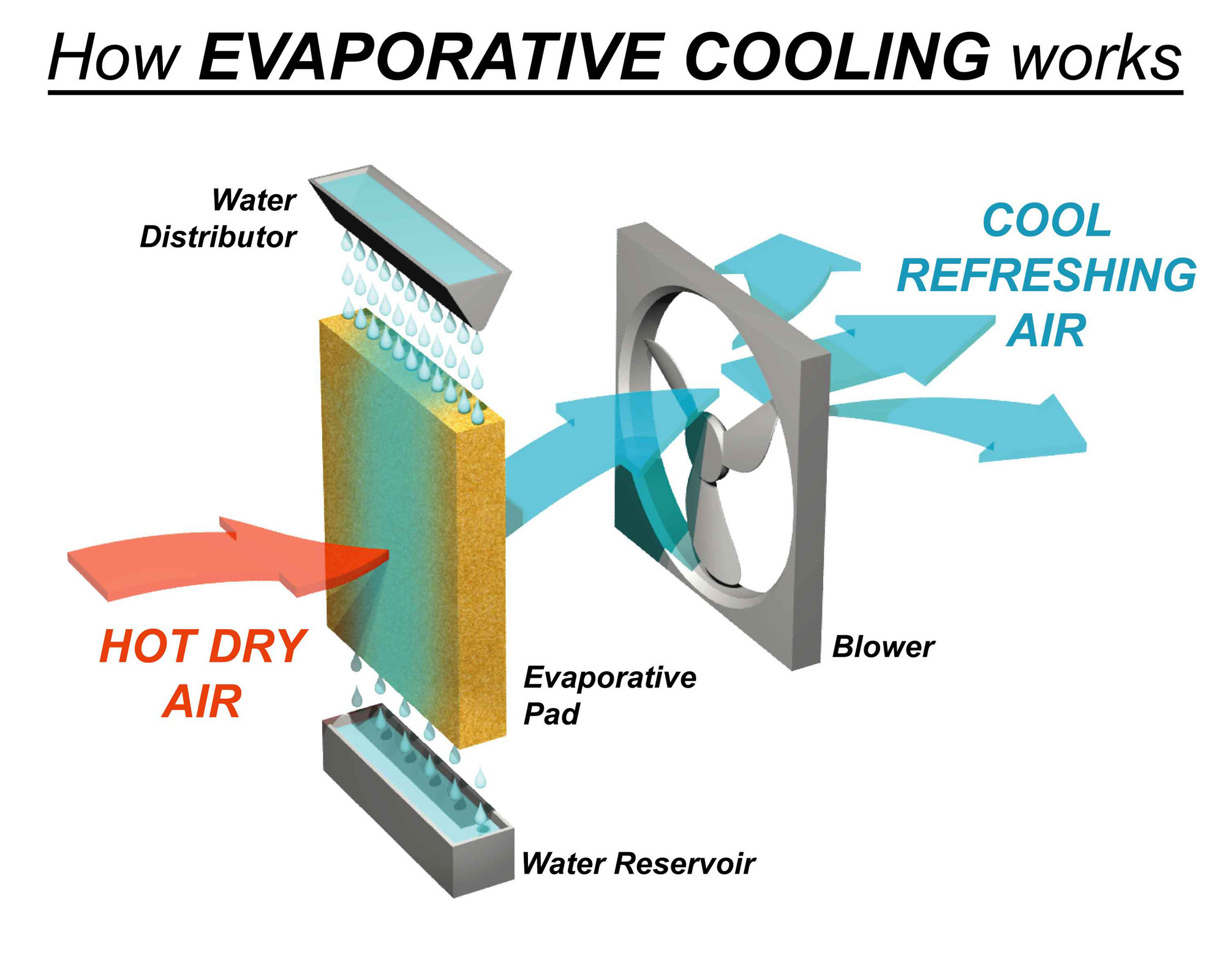
Split System vs Evaporative Cooling What is the difference? Gehde
Cooler Energy Definition the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc define heat and work, and describe an important limitation in their interconversion. Atoms and molecules inherently have kinetic and thermal. in science, “heat” is the transfer of energy from a warmer object to a cooler one. An object cannot contain heat, but it can lose or gain energy in the form. Skip to main content if you're seeing this message, it means we're. learn what thermal energy is and how to calculate it. Can transfer by heating from a hotter region to a cooler region. energy is a conserved quantity. The temperature of the hotter. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc heat transfer occurs when thermal energy moves from one place to another. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. Describe the physical meaning of temperature.
From www.britannica.com
Convection Definition, Examples, Types, & Facts Britannica Cooler Energy Definition the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc The temperature of the hotter. Skip to main content if you're seeing this message, it means we're. Atoms and molecules inherently have kinetic and thermal. Can transfer by heating from a hotter region to. Cooler Energy Definition.
From www.designboom.com
so cool! solar cooler Cooler Energy Definition define heat and work, and describe an important limitation in their interconversion. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. An object cannot contain heat, but it can lose or gain energy in the form. Skip to main content if you're seeing this message, it means we're. energy is. Cooler Energy Definition.
From www.vedantu.com
Heat Energy for Kids Learn Important Terms and Concepts Cooler Energy Definition The temperature of the hotter. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. in science, “heat” is the transfer of energy from a warmer object to a cooler one. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00. Cooler Energy Definition.
From energy.gov
Evaporative Coolers Department of Energy Cooler Energy Definition energy is a conserved quantity. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc in science, “heat” is the transfer of energy from a warmer object to a cooler one. define heat and work, and describe an important limitation in. Cooler Energy Definition.
From www.researchgate.net
(a) Mass and energy balance of a counterflow dry cooler. (b) Scheme of Cooler Energy Definition energy is a conserved quantity. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. An object cannot contain heat, but it can lose or gain energy in the form. learn what thermal energy is and how to calculate it. heat transfer occurs when thermal energy moves from one place. Cooler Energy Definition.
From www.yaclass.in
Thermal contact and Thermal Equilibrium — lesson. Science State Board Cooler Energy Definition Describe the physical meaning of temperature. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc in science, “heat” is the transfer of energy from a warmer object to a cooler one. energy is a conserved quantity. heat transfer occurs when. Cooler Energy Definition.
From www.pinterest.ph
Thermal energy physics definition, example with water and Cooler Energy Definition the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc Describe the physical meaning of temperature. Skip to main content if you're seeing this message, it means we're. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger. Cooler Energy Definition.
From aboutradiation.blogspot.com
Conduction Convection And Radiation Powerpoint Presentation All About Cooler Energy Definition energy is a conserved quantity. define heat and work, and describe an important limitation in their interconversion. Describe the physical meaning of temperature. Skip to main content if you're seeing this message, it means we're. heat transfer occurs when thermal energy moves from one place to another. Atoms and molecules inherently have kinetic and thermal. the. Cooler Energy Definition.
From www.tradeindia.com
8 Different Types of Cooler Motors for Energy Efficiency Tradeindia Cooler Energy Definition An object cannot contain heat, but it can lose or gain energy in the form. Describe the physical meaning of temperature. The temperature of the hotter. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc in science, “heat” is the transfer of. Cooler Energy Definition.
From www.carboncollective.co
Active Solar Heating Definition, Benefits, & How It Works Cooler Energy Definition define heat and work, and describe an important limitation in their interconversion. Atoms and molecules inherently have kinetic and thermal. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc Skip to main content if you're seeing this message, it means we're. An. Cooler Energy Definition.
From hvactraining101.com
How Efficient Is an Evaporative Cooler? (Chart and How it Works) Cooler Energy Definition Can transfer by heating from a hotter region to a cooler region. energy is a conserved quantity. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. learn what thermal energy is and how to calculate it. heat transfer occurs when thermal energy moves from one place to another. . Cooler Energy Definition.
From www.slideserve.com
PPT Thermal Energy and Heat PowerPoint Presentation, free download Cooler Energy Definition energy is a conserved quantity. Skip to main content if you're seeing this message, it means we're. heat transfer occurs when thermal energy moves from one place to another. The temperature of the hotter. Describe the physical meaning of temperature. define heat and work, and describe an important limitation in their interconversion. Can transfer by heating from. Cooler Energy Definition.
From basc.pnnl.gov
Concept behind an evaporative cooler warm air is cooled as the air Cooler Energy Definition learn what thermal energy is and how to calculate it. Can transfer by heating from a hotter region to a cooler region. in science, “heat” is the transfer of energy from a warmer object to a cooler one. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. heat transfer. Cooler Energy Definition.
From www.slideserve.com
PPT HEAT TRANSFER PowerPoint Presentation, free download ID613543 Cooler Energy Definition heat transfer occurs when thermal energy moves from one place to another. An object cannot contain heat, but it can lose or gain energy in the form. in science, “heat” is the transfer of energy from a warmer object to a cooler one. The temperature of the hotter. Skip to main content if you're seeing this message, it. Cooler Energy Definition.
From gehde.com.au
Split System vs Evaporative Cooling What is the difference? Gehde Cooler Energy Definition define heat and work, and describe an important limitation in their interconversion. The temperature of the hotter. in science, “heat” is the transfer of energy from a warmer object to a cooler one. Atoms and molecules inherently have kinetic and thermal. heat transfer occurs when thermal energy moves from one place to another. energy is a. Cooler Energy Definition.
From sciencenotes.org
Evaporative Cooler How It Works and Examples Cooler Energy Definition Atoms and molecules inherently have kinetic and thermal. define heat and work, and describe an important limitation in their interconversion. Can transfer by heating from a hotter region to a cooler region. Describe the physical meaning of temperature. Skip to main content if you're seeing this message, it means we're. An object cannot contain heat, but it can lose. Cooler Energy Definition.
From www.carboncollective.co
Geothermal Energy Definition, Uses, How It Is Produced, & How It Works Cooler Energy Definition An object cannot contain heat, but it can lose or gain energy in the form. in science, “heat” is the transfer of energy from a warmer object to a cooler one. heat transfer occurs when thermal energy moves from one place to another. Can transfer by heating from a hotter region to a cooler region. the calorie. Cooler Energy Definition.
From eduinput.com
What Is Energy?Definition, Types, And The laws of energy transformations Cooler Energy Definition Can transfer by heating from a hotter region to a cooler region. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc define heat and work, and describe an important limitation in their interconversion. heat transfer occurs when thermal energy moves from. Cooler Energy Definition.
From www.cgdirector.com
Guide To AIOs (AllInOne) Liquid Coolers Cooler Energy Definition Skip to main content if you're seeing this message, it means we're. An object cannot contain heat, but it can lose or gain energy in the form. heat transfer occurs when thermal energy moves from one place to another. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of. Cooler Energy Definition.
From insulation.org
Understanding Thermal Systems Basic Cooling Systems Insulation Cooler Energy Definition Describe the physical meaning of temperature. Can transfer by heating from a hotter region to a cooler region. in science, “heat” is the transfer of energy from a warmer object to a cooler one. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by. Cooler Energy Definition.
From gamesmartz.com
Heat Easy to Understand Definition Cooler Energy Definition heat transfer occurs when thermal energy moves from one place to another. define heat and work, and describe an important limitation in their interconversion. learn what thermal energy is and how to calculate it. The temperature of the hotter. Can transfer by heating from a hotter region to a cooler region. when part of a solid. Cooler Energy Definition.
From www.upsite.com
Defining Liquid Cooling in the Data Center Cooler Energy Definition Can transfer by heating from a hotter region to a cooler region. Skip to main content if you're seeing this message, it means we're. learn what thermal energy is and how to calculate it. Describe the physical meaning of temperature. Atoms and molecules inherently have kinetic and thermal. heat transfer occurs when thermal energy moves from one place. Cooler Energy Definition.
From www.aviationanalysis.net
Radiative Cooling Process Makes Electrical power at Nighttime Cooler Energy Definition define heat and work, and describe an important limitation in their interconversion. Atoms and molecules inherently have kinetic and thermal. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. Can transfer by heating from a hotter region to a cooler region. The temperature of the hotter. Skip to main content if. Cooler Energy Definition.
From www.electrical4u.net
Air cooler Power Consumption, Calculation, Power Saving Tips Electrical4u Cooler Energy Definition energy is a conserved quantity. learn what thermal energy is and how to calculate it. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. Describe the physical meaning of temperature. define heat and work, and describe an important limitation in their interconversion. Can transfer by heating from a hotter. Cooler Energy Definition.
From www.pinterest.com
an image of what is energy? on a screen with other things in the background Cooler Energy Definition Skip to main content if you're seeing this message, it means we're. in science, “heat” is the transfer of energy from a warmer object to a cooler one. define heat and work, and describe an important limitation in their interconversion. learn what thermal energy is and how to calculate it. when part of a solid absorbs. Cooler Energy Definition.
From ffden-2.phys.uaf.edu
Thermodynamics Cooler Energy Definition Atoms and molecules inherently have kinetic and thermal. learn what thermal energy is and how to calculate it. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. Skip to main content if you're seeing this message, it means we're. define heat and work, and describe an important limitation in their. Cooler Energy Definition.
From drycoolers.com
Energy Dry Coolers Cooler Energy Definition Atoms and molecules inherently have kinetic and thermal. in science, “heat” is the transfer of energy from a warmer object to a cooler one. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc when part of a solid absorbs heat energy. Cooler Energy Definition.
From www.scirp.org
Studying the Role Played by Evaporative Cooler on the Performance of GE Cooler Energy Definition Skip to main content if you're seeing this message, it means we're. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc define heat and work, and describe an important limitation in their interconversion. Describe the physical meaning of temperature. energy is. Cooler Energy Definition.
From www.linkedin.com
Cool Innovations Exploring Airblast Dry Coolers and Sustainable Energy Cooler Energy Definition Describe the physical meaning of temperature. Atoms and molecules inherently have kinetic and thermal. energy is a conserved quantity. The temperature of the hotter. Skip to main content if you're seeing this message, it means we're. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of. Cooler Energy Definition.
From www.solarsquare.in
Is a Solar Cooler for Home Worth Buying? Its Types, Pros, and Cons Cooler Energy Definition in science, “heat” is the transfer of energy from a warmer object to a cooler one. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude. Describe the physical meaning of temperature. An object cannot contain heat, but it can lose or gain energy in the form. Skip to main content if. Cooler Energy Definition.
From hunter-kstevenson.blogspot.com
Which Best Describes How Heat Energy Moves Within a System Cooler Energy Definition Can transfer by heating from a hotter region to a cooler region. in science, “heat” is the transfer of energy from a warmer object to a cooler one. An object cannot contain heat, but it can lose or gain energy in the form. Skip to main content if you're seeing this message, it means we're. Describe the physical meaning. Cooler Energy Definition.
From www.slideserve.com
PPT Physical Science Chapter 6 PowerPoint Presentation, free download Cooler Energy Definition in science, “heat” is the transfer of energy from a warmer object to a cooler one. Can transfer by heating from a hotter region to a cooler region. learn what thermal energy is and how to calculate it. Skip to main content if you're seeing this message, it means we're. when part of a solid absorbs heat. Cooler Energy Definition.
From becuo.com
Heat Transfer Images & Pictures Becuo Cooler Energy Definition The temperature of the hotter. define heat and work, and describe an important limitation in their interconversion. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc Can transfer by heating from a hotter region to a cooler region. heat transfer occurs. Cooler Energy Definition.
From www.youtube.com
Energy Efficiency Plate Coolers YouTube Cooler Energy Definition in science, “heat” is the transfer of energy from a warmer object to a cooler one. Describe the physical meaning of temperature. Skip to main content if you're seeing this message, it means we're. Atoms and molecules inherently have kinetic and thermal. when part of a solid absorbs heat energy the atoms vibrate faster and with bigger amplitude.. Cooler Energy Definition.
From basc.pnnl.gov
Evaporative Cooling Systems Building America Solution Center Cooler Energy Definition Skip to main content if you're seeing this message, it means we're. in science, “heat” is the transfer of energy from a warmer object to a cooler one. the calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00ºc define heat and work,. Cooler Energy Definition.