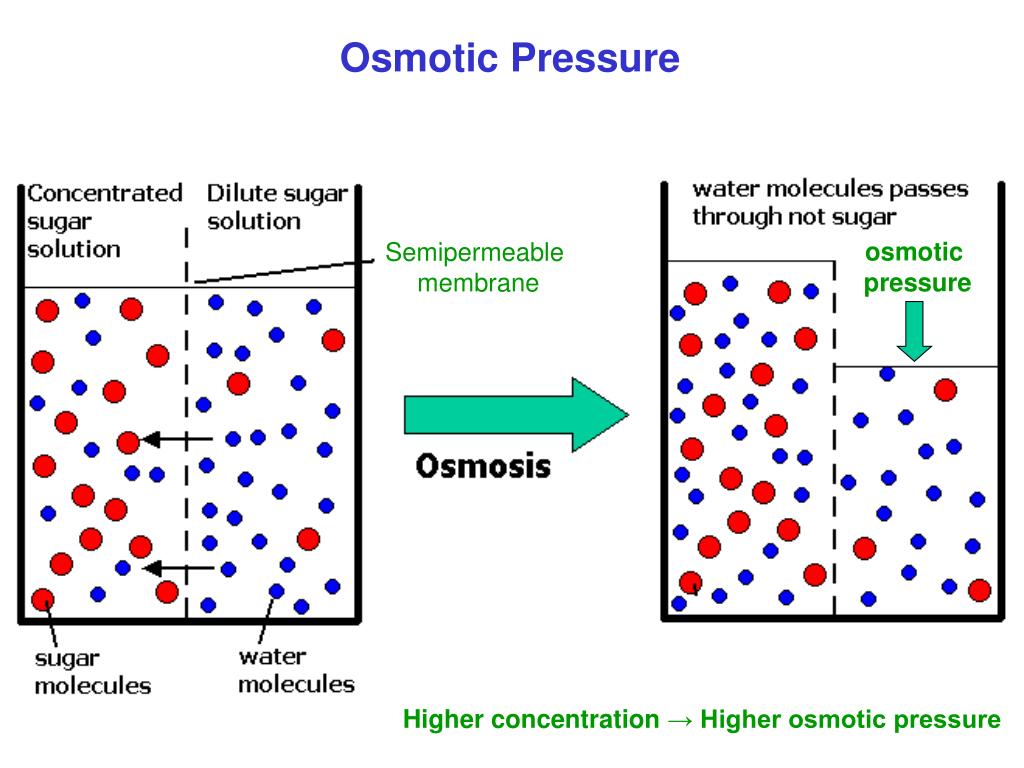
Colloid Solutions Effects . A good example is the way a glass of skim milk (a. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). Crystalloids have small molecules, are cheap, easy to. the properties of colloids differ from those observed in true solutions or suspensions. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. For instance, they often display unique optical effects, such as. colloid or crystalloid solutions may be used for this purpose. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and.
from www.slideserve.com
the properties of colloids differ from those observed in true solutions or suspensions. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. For instance, they often display unique optical effects, such as. A good example is the way a glass of skim milk (a. colloid or crystalloid solutions may be used for this purpose. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). Crystalloids have small molecules, are cheap, easy to. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion.
PPT Chapter 6 Solutions and Colloids PowerPoint Presentation, free download ID5743281
Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. Crystalloids have small molecules, are cheap, easy to. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. For instance, they often display unique optical effects, such as. A good example is the way a glass of skim milk (a. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. the properties of colloids differ from those observed in true solutions or suspensions. colloid or crystalloid solutions may be used for this purpose. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Chemistry Aakash Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. A good example is the way a glass of skim milk (a. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). Crystalloids have small molecules, are cheap, easy to. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate. Colloid Solutions Effects.
From www.thoughtco.com
Tyndall Effect Definition and Examples Colloid Solutions Effects a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. For instance, they often display unique optical effects, such as. in this chapter, we will consider the nature of solutions, and examine factors that determine whether. Colloid Solutions Effects.
From www.shutterstock.com
Tyndall Effect Colloidal Solution Light Beam 库存矢量图(免版税)391233595 Shutterstock Colloid Solutions Effects A good example is the way a glass of skim milk (a. For instance, they often display unique optical effects, such as. the properties of colloids differ from those observed in true solutions or suspensions. Crystalloids have small molecules, are cheap, easy to. in this chapter, we will consider the nature of solutions, and examine factors that determine. Colloid Solutions Effects.
From www.youtube.com
Science 9 Chapter 2 Suspension, Colloid, Tyndall effect, Comparison of Solution Colloid Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. A good example is the way a glass of skim milk (a. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). the properties of. Colloid Solutions Effects.
From www.youtube.com
Solutions Suspensions and Colloids Part 1/1 English Class 9 YouTube Colloid Solutions Effects the properties of colloids differ from those observed in true solutions or suspensions. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. Crystalloids have small molecules, are cheap, easy to. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of.. Colloid Solutions Effects.
From www.slideserve.com
PPT Solutions and Colloidal Dispersions PowerPoint Presentation, free download ID2209641 Colloid Solutions Effects the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. A good example is the way a glass of skim milk (a. Crystalloids have small molecules, are cheap, easy to. in this chapter, we will. Colloid Solutions Effects.
From exouqcyfe.blob.core.windows.net
What Is Colloidal Suspension In Chemistry at Robert Lemond blog Colloid Solutions Effects in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. For instance, they often display unique optical effects, such as. A good example is the way a glass of skim milk (a. Crystalloids have small molecules, are cheap, easy to. the properties of colloids differ from those. Colloid Solutions Effects.
From testbook.com
ColloidsMeaning, Classification, Properties, Example and Notes Colloid Solutions Effects a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. For instance, they often display unique optical effects, such as. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. colloid or crystalloid solutions may be used for this purpose. Crystalloids. Colloid Solutions Effects.
From surfguppy.com
Properties of Colloids Surfguppy Chemistry made easy visual learning Colloid Solutions Effects For instance, they often display unique optical effects, such as. Crystalloids have small molecules, are cheap, easy to. A good example is the way a glass of skim milk (a. colloid or crystalloid solutions may be used for this purpose. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). a group. Colloid Solutions Effects.
From slideplayer.com
Physical Properties of Solutions ppt download Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). the tyndall effect is the scattering of light by the particles in a colloid or fine. Colloid Solutions Effects.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Colloid Solutions Effects the properties of colloids differ from those observed in true solutions or suspensions. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. colloid or crystalloid solutions may be used for this purpose. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). in this. Colloid Solutions Effects.
From www.semanticscholar.org
Figure 2 from The Effects of Colloid Solutions on Renal Proximal Tubular Cells In Vitro Colloid Solutions Effects a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. Crystalloids have small molecules, are cheap, easy to. A. Colloid Solutions Effects.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of solution 21669338 Vector Colloid Solutions Effects explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. colloids and. Colloid Solutions Effects.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Solutions Effects in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. the properties of. Colloid Solutions Effects.
From exoqmcvtq.blob.core.windows.net
Colloid Solution Osmotic at Ilene Wang blog Colloid Solutions Effects in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. a group. Colloid Solutions Effects.
From www.slideserve.com
PPT Chapter 6 Solutions and Colloids PowerPoint Presentation, free download ID5743281 Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. the properties of colloids differ from those observed in true solutions or suspensions. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. A good example is the way a glass of skim milk (a. a group of mixtures. Colloid Solutions Effects.
From www.istockphoto.com
Tyndall Effect Is Scattering Of Light In Colloid Mixtures Stock Illustration Download Image Colloid Solutions Effects Crystalloids have small molecules, are cheap, easy to. A good example is the way a glass of skim milk (a. colloid or crystalloid solutions may be used for this purpose. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. the tyndall effect is the scattering of light by the particles in. Colloid Solutions Effects.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID2012080 Colloid Solutions Effects in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). Crystalloids have small molecules, are. Colloid Solutions Effects.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Colloid Solutions Effects colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). Crystalloids have small molecules, are cheap, easy to. colloid or crystalloid solutions may be used for this purpose. A good example is the way a glass of skim milk (a. For instance, they often display unique optical effects, such as. a group. Colloid Solutions Effects.
From uen.pressbooks.pub
Colloids Introductory Chemistry Colloid Solutions Effects explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. Crystalloids have small molecules, are cheap, easy to. A good example is the way a glass of skim milk (a. the tyndall effect is the scattering. Colloid Solutions Effects.
From www.semanticscholar.org
Figure 1 from The Effects of Colloid Solutions on Renal Proximal Tubular Cells In Vitro Colloid Solutions Effects A good example is the way a glass of skim milk (a. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. For instance, they often display unique optical effects, such as. the properties of. Colloid Solutions Effects.
From chemistrytalk.org
What Are Colloids? ChemTalk Colloid Solutions Effects explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. Crystalloids have small molecules, are cheap, easy to. colloid or crystalloid solutions may be used for this purpose. For instance, they often display unique optical effects, such as. the tyndall effect is the scattering of light by the particles in a. Colloid Solutions Effects.
From dxofmeeyi.blob.core.windows.net
Colloid Solutions Nursing at Bernard Oyola blog Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. A good example is the way a glass of skim milk (a. the properties of colloids differ from those observed in true solutions or suspensions. in this chapter, we will consider. Colloid Solutions Effects.
From www.yaclass.in
Tyndall effect and Raman scattering — lesson. Science State Board, Class 10. Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. Crystalloids have small molecules, are cheap, easy to. A good example is the way a glass of skim milk (a. For instance, they often display unique optical effects, such as. explain why. Colloid Solutions Effects.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True solutions Colloids Suspensions Colloid Solutions Effects explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. A good example is the way a glass of. Colloid Solutions Effects.
From www.youtube.com
Colloids The Tyndall Effect (H82INC) YouTube Colloid Solutions Effects colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). Crystalloids have small molecules, are cheap, easy to. the properties of colloids differ from those observed in true solutions or suspensions. colloid or crystalloid solutions may be used for this purpose. the tyndall effect is the scattering of light by the. Colloid Solutions Effects.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download ID822807 Colloid Solutions Effects in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. Crystalloids have small molecules, are cheap, easy to. colloid or crystalloid solutions may be used for this purpose. the. Colloid Solutions Effects.
From spectrumcentre.netlify.app
The scattering of light in a colloid Colloid Solutions Effects in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. For instance, they often. Colloid Solutions Effects.
From slideplayer.com
Solutions and Colloids ppt download Colloid Solutions Effects a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. Crystalloids have small molecules, are cheap, easy to. explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. the properties of colloids differ from those observed in true solutions or suspensions. colloid or crystalloid solutions. Colloid Solutions Effects.
From dxofmeeyi.blob.core.windows.net
Colloid Solutions Nursing at Bernard Oyola blog Colloid Solutions Effects colloid or crystalloid solutions may be used for this purpose. For instance, they often display unique optical effects, such as. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. the properties of colloids differ from those observed in true solutions or suspensions. explain why freezing or addition of. Colloid Solutions Effects.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID6630187 Colloid Solutions Effects a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. For instance, they often display unique optical effects, such as. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. the properties of colloids differ from those observed in true solutions. Colloid Solutions Effects.
From collegedunia.com
Classification of Colloids Properties & Phases Colloid Solutions Effects explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. the tyndall effect is the scattering of light by the particles in a colloid or fine suspension. For instance, they often display unique optical effects, such as. colloids and crystalloids are two types of solutions used to replace lost blood fluid. Colloid Solutions Effects.
From www.researchgate.net
(PDF) Effects of colloid solutions on ischemiareperfusioninduced periosteal microcirculatory Colloid Solutions Effects the properties of colloids differ from those observed in true solutions or suspensions. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). A good example is the way a glass of skim milk (a. colloid or crystalloid solutions may be used for this purpose. a group of mixtures called colloids. Colloid Solutions Effects.
From www.researchgate.net
(PDF) Depletion effects in colloidpolymer solutions Colloid Solutions Effects explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion. For instance, they often display unique optical effects, such as. A good example is the way a glass of skim milk (a. colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). the tyndall effect is. Colloid Solutions Effects.
From www.geeksforgeeks.org
Colloids Definition, Classification, Application, Properties, & FAQs Colloid Solutions Effects For instance, they often display unique optical effects, such as. Crystalloids have small molecules, are cheap, easy to. A good example is the way a glass of skim milk (a. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. the properties of colloids differ from those. Colloid Solutions Effects.