Bromine Chloride Equation . To balance a chemical equation, enter an equation of a chemical. Bromine (i) chloride is a chloride of bromine. Chemical equation (h2o = h + o) 🛠️. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Bromine + chlorine = bromine monochloride. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Cl 2 (g) + 2kbr(aq) →.
from studylib.net
To balance a chemical equation, enter an equation of a chemical. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Bromine + chlorine = bromine monochloride. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Cl 2 (g) + 2kbr(aq) →. Chemical equation (h2o = h + o) 🛠️. Bromine (i) chloride is a chloride of bromine. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water.
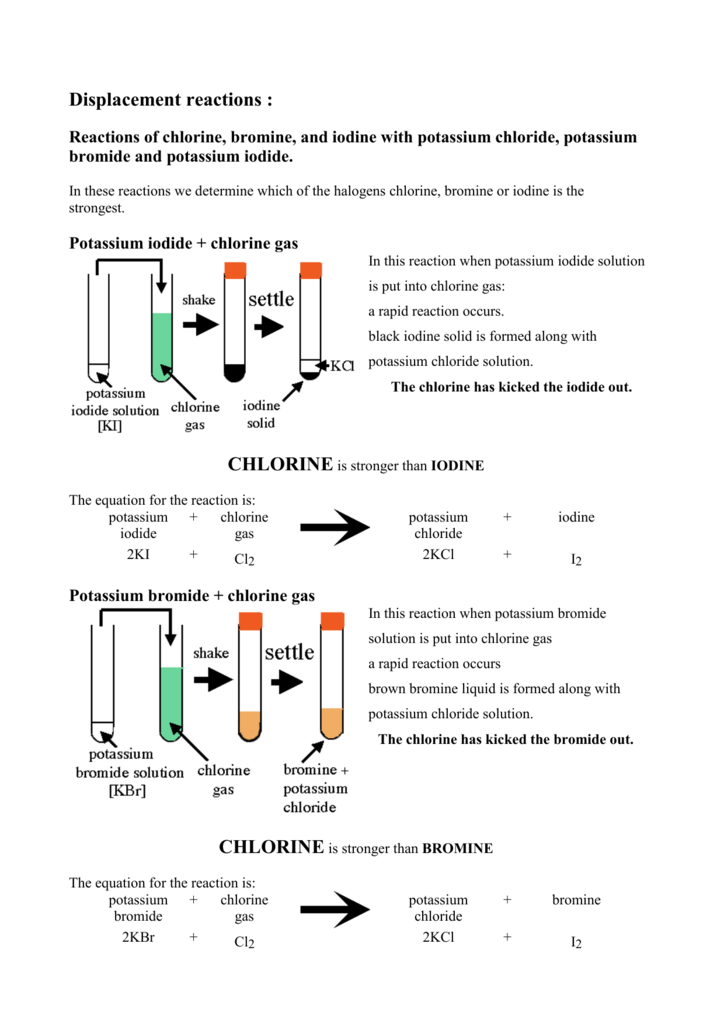
Displacement reactions Reactions of chlorine
Bromine Chloride Equation Chemical equation (h2o = h + o) 🛠️. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Bromine (i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. To balance a chemical equation, enter an equation of a chemical. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (g) + 2kbr(aq) →. Chemical equation (h2o = h + o) 🛠️. Bromine + chlorine = bromine monochloride.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Bromine Chloride Equation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Bromine + chlorine = bromine monochloride. Bromine (i) chloride is a chloride of bromine. Chlorine + potassium bromide → bromine. Bromine Chloride Equation.
From brainly.com
Balance the chemical equation. Based on the equation, how many grams of Bromine Chloride Equation Chemical equation (h2o = h + o) 🛠️. Bromine + chlorine = bromine monochloride. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (aq) + 2ki (aq). Bromine Chloride Equation.
From www.numerade.com
SOLVED 'QUESTION 8 Aluminum will react with bromine to form aluminum Bromine Chloride Equation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Bromine + chlorine = bromine monochloride. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Cl 2 (g) + 2kbr(aq) →. Cl 2 (aq) + 2ki (aq) → 2kcl. Bromine Chloride Equation.
From www.numerade.com
SOLVED According to the following reaction, how many grams of chlorine Bromine Chloride Equation Cl 2 (g) + 2kbr(aq) →. Bromine + chlorine = bromine monochloride. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Bromine (i) chloride is a. Bromine Chloride Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine Chloride Equation To balance a chemical equation, enter an equation of a chemical. Bromine (i) chloride is a chloride of bromine. Bromine + chlorine = bromine monochloride. Cl 2 (g) + 2kbr(aq) →. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Chemical equation (h2o = h + o) 🛠️. It is used as a biocide, specifically as an algaecide,. Bromine Chloride Equation.
From studylib.net
Displacement reactions Reactions of chlorine Bromine Chloride Equation Chemical equation (h2o = h + o) 🛠️. Bromine + chlorine = bromine monochloride. To balance a chemical equation, enter an equation of a chemical. Bromine (i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Cl 2 (g) + 2kbr(aq) →. When chlorine (as. Bromine Chloride Equation.
From brainly.com
Chlorine gas reacts with aqueous sodium bromide to produce aqueous Bromine Chloride Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Bromine + chlorine = bromine monochloride. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. When chlorine (as a. Bromine Chloride Equation.
From www.toppr.com
When phenol is treated with bromine water, while precipitate is Bromine Chloride Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling. Bromine Chloride Equation.
From animalia-life.club
Iodine Solid Formula Bromine Chloride Equation Bromine (i) chloride is a chloride of bromine. Cl 2 (g) + 2kbr(aq) →. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + potassium. Bromine Chloride Equation.
From www.numerade.com
SOLVED (ii) Aniline reacts with bromine water in aqueous medium (only Bromine Chloride Equation Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Bromine (i) chloride is a chloride of bromine. Bromine + chlorine = bromine monochloride. To balance a chemical equation, enter an equation of a chemical. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. When chlorine (as. Bromine Chloride Equation.
From saniyahghopdalton.blogspot.com
Reaction of Cyclohexene With Bromine Bromine Chloride Equation Cl 2 (g) + 2kbr(aq) →. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. To balance a chemical equation, enter an equation of a chemical. Bromine (i) chloride is a chloride of bromine. Chemical equation (h2o = h + o) 🛠️. Br + cl = brcl. Bromine Chloride Equation.
From www.numerade.com
SOLVEDThe first preparation of chlorine was similar to the synthesis Bromine Chloride Equation Chemical equation (h2o = h + o) 🛠️. Bromine + chlorine = bromine monochloride. Bromine (i) chloride is a chloride of bromine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the. Bromine Chloride Equation.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Bromine Chloride Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. To balance a chemical equation, enter an equation of a chemical. Bromine (i) chloride is a. Bromine Chloride Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Bromine Chloride Equation Bromine (i) chloride is a chloride of bromine. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chemical equation (h2o = h + o) 🛠️. It. Bromine Chloride Equation.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Bromine Chloride Equation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Chemical equation (h2o = h + o) 🛠️. To balance a chemical equation, enter an equation of a chemical. When chlorine. Bromine Chloride Equation.
From chemistry291.blogspot.com
Chemical Formula for Sodium ChlorideSodium Chloride Formula Bromine Chloride Equation Chemical equation (h2o = h + o) 🛠️. Bromine (i) chloride is a chloride of bromine. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. When chlorine (as a gas. Bromine Chloride Equation.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum Bromine Chloride Equation To balance a chemical equation, enter an equation of a chemical. Cl 2 (g) + 2kbr(aq) →. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Cl 2 (aq) +. Bromine Chloride Equation.
From www.coursehero.com
[Solved] REACTION OF ALCOHOL AND PHENOL REACTION OF PHENOL PART E Bromine Chloride Equation Cl 2 (g) + 2kbr(aq) →. Bromine + chlorine = bromine monochloride. Chemical equation (h2o = h + o) 🛠️. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. To balance a chemical equation, enter an equation of a chemical. It is used as a biocide, specifically as. Bromine Chloride Equation.
From www.youtube.com
Reaction of Sodium (Na) with Bromine (Br2) YouTube Bromine Chloride Equation Bromine + chlorine = bromine monochloride. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: To balance a chemical equation, enter an equation of a chemical. When chlorine (as a gas or dissolved in water) is added. Bromine Chloride Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine Chloride Equation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. To balance a chemical equation, enter an equation of a chemical. Chemical equation (h2o = h + o) 🛠️. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of. Bromine Chloride Equation.
From www.numerade.com
SOLVED Word equation and balanced equation for aqueous magnesium Bromine Chloride Equation Bromine (i) chloride is a chloride of bromine. Chemical equation (h2o = h + o) 🛠️. Bromine + chlorine = bromine monochloride. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (g) + 2kbr(aq) →. It is used as a biocide, specifically as an algaecide,. Bromine Chloride Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NaBr + Cl2 = NaCl + Br2 YouTube Bromine Chloride Equation Cl 2 (g) + 2kbr(aq) →. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. To balance a chemical equation, enter an equation of a chemical. Chemical equation (h2o = h + o) 🛠️. Bromine + chlorine = bromine monochloride. Chlorine + potassium bromide → bromine +. Bromine Chloride Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine Chloride Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. To balance a chemical equation, enter an equation of a chemical. Chemical equation (h2o = h + o) 🛠️. Bromine (i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and. Bromine Chloride Equation.
From www.numerade.com
SOLVED Q4 The iodide in the sample that also contained chloride was Bromine Chloride Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Bromine + chlorine = bromine monochloride. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: To balance a chemical equation, enter an equation of a chemical. It is used as a biocide, specifically as an algaecide, fungicide,. Bromine Chloride Equation.
From www.numerade.com
SOLVED 5. Write a chemical equation for the reaction of acetylene with Bromine Chloride Equation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Bromine (i) chloride is a chloride of bromine. Cl 2. Bromine Chloride Equation.
From www.numerade.com
SOLVED TABLE 13.1 Representative Electrophilic Aromatic Substitution Bromine Chloride Equation Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Cl 2 (g) + 2kbr(aq) →. To balance a chemical equation, enter an equation of a chemical. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Bromine (i) chloride is a chloride of bromine. Bromine +. Bromine Chloride Equation.
From www.slideshare.net
Chemical Reactions Bromine Chloride Equation To balance a chemical equation, enter an equation of a chemical. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Bromine + chlorine = bromine monochloride. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of. Bromine Chloride Equation.
From www.numerade.com
SOLVEDThe nonspontaneous redox reaction of bromine and aqueous sodium Bromine Chloride Equation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Bromine + chlorine = bromine monochloride. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution. Bromine Chloride Equation.
From brainly.com
When aqueous magnesium chloride reacts with liquid bromine Bromine Chloride Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. To balance a chemical equation, enter an equation of a chemical. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. It is used as a biocide,. Bromine Chloride Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine Chloride Equation When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Cl 2 (g) + 2kbr(aq) →. To balance a chemical equation, enter an equation of a chemical. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution. Bromine Chloride Equation.
From www.tessshebaylo.com
Net Ionic Equation For Ammonium Chloride And Water Tessshebaylo Bromine Chloride Equation It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. To balance a chemical equation, enter an equation of a chemical. Bromine + chlorine = bromine monochloride. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Br + cl. Bromine Chloride Equation.
From en.ppt-online.org
Electrolysis online presentation Bromine Chloride Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Chemical equation (h2o = h. Bromine Chloride Equation.
From www.youtube.com
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas Bromine Chloride Equation Chemical equation (h2o = h + o) 🛠️. Chlorine + potassium bromide → bromine + potassium chloride symbol equation: Bromine (i) chloride is a chloride of bromine. Cl 2 (g) + 2kbr(aq) →. Bromine + chlorine = bromine monochloride. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of. Bromine Chloride Equation.
From www.numerade.com
SOLVED Chlorine can replace bromine in bromide compounds, forming Bromine Chloride Equation Chemical equation (h2o = h + o) 🛠️. Bromine + chlorine = bromine monochloride. Bromine (i) chloride is a chloride of bromine. To balance a chemical equation, enter an equation of a chemical. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Cl 2 (g) + 2kbr(aq) →.. Bromine Chloride Equation.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Bromine Chloride Equation Bromine (i) chloride is a chloride of bromine. Chemical equation (h2o = h + o) 🛠️. Cl 2 (g) + 2kbr(aq) →. To balance a chemical equation, enter an equation of a chemical. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Bromine + chlorine = bromine. Bromine Chloride Equation.